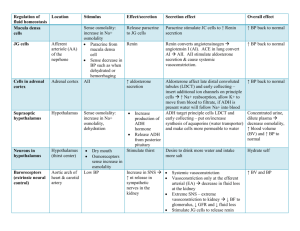
Renal21-AcidBaseBalanceII
advertisement

Renal 21 Thur 03/20/03 9am Dr. Mallet Le Giang Proscribe Samera Kasim Page 1 of 7 Acid Base Balance II Cont from 8am lecture…. I. Excretion of H+ (figure) a. 2 options: b. as a titratable acid i. (berne & levy fig 44-4, p. 769) ii. CO2 and water move into the cell, and carbonic anhydrase combines them, making carbonic acid, which further dissociates into H+ and HCO3-. This results in the secretion of H+. iii. H+ combines with a filtered base such as HPO4, forming the conjugate acid of that base, H2PO4. iv. This is excreted in the urine v. Bicarb is made in the kidneys from CO2 and H20 and returned to the body vi. This bicarb is used to replenish that used to buffer the H+ secreted. vii. SO, an acid is being made somewhere in the body, increasing H+ conc. Bicarb buffers it, resulting in decr [bicarb]. When the kidneys excrete the acid made, they actually make bicarb to replace that which did the buffering. c. as ammonium (NH4) i. (Berne & Levy Fig 44-5, p. 770) ii. ammonium is not filtered, but is generated from the metabolism of glutamine, which is an important energy source in the renal tubular epithelium. iii. When oxidized, it releases 2 ammonia, which combine with 2 H+ to make ammonium, which carries a + charge. Thus if it leaves the cell, it is exchanged with Na. iv. OR the ammonia could diffuse through the lipid bilayer to the lumen to form ammonium. v. This is the major way by which the kidneys eliminate fixed acids from the body. vi. The rate at which this occurs can be adjusted as in chronic acidemia II. Chronic acidemia (graph) i. In chronic acidemia, the kidneys up-regulate their ability to oxidize glutamine and make ammonium, thus resulting in increased urine ammonium at a given pH. ii. Chronic academia also activates the genetic machinery that stimulates the synthesis of glutaminase, which is the enzyme that catalyzes the rate limiting step in glutamine. More glutaminase Renal 21 Thur 03/20/03 9am Dr. Mallet Le Giang Proscribe Samera Kasim Page 2 of 7 increases the ability for the oxidation of glutamine and subsequent release of the amino groups to make ammonium. III. SUMMARY: Session 8 a. Henderson-Hasselbalch equation b. First line of defense: buffer systems c. Bicarbonate system is an ‘open’ buffer system d. Second line of defense: ventilation (lungs remove acid by removing CO2) e. Third line of defense: renal excretion of H+ f. Bicarbonate reabsorption adjustment g. Excretion of H+ as titratable acids h. Excretion of H+ as ammonium i. Very little of free acid secreted as the free H+ ion. IV. OVERVIEW: Session 9 a. Physiological control of renal H+ secretion: six factors influence epithelial H+ secretion b. Mechanisms that defend intracellular pH throughout the body c. Acid-base disorders d. Respiratory acidosis, respiratory alkalosis (change in PCO2) e. Metabolic acidosis, metabolic alkalosis f. Anion gap: differential diagnosis of metabolic acidosis i. KNOW THIS V. Alkalemia a. collecting ducts secrete HCO3b. β-Intercalated cells actively secrete bicarb into the lumen VI. Factors controlling renal H+ secretion a. There’s 6 of them… b. High H+ concentration in the cell means there’s more available to secrete c. PCO2 in the blood: as the partial pressure of CO2 increases during pulmonary insufficiency, CO2 can diffuse into the blood, where it combines with water to form H+ will. i. CO2 is a source of H+ via the carbonic anhydrase reaction. d. Carbonic Anhydrase (CA) activity: CA is an abundant enzyme; diuretics can inhibit them and impede the reaction: CO2 + H2O H2CO3 i. Normally not a limiting factor; it’s an abundant enzyme in the tubular epithelium except in the presence of Carbonic anhydrase inhibiting diuretics ii. Tubular H+ secretions decrease, predisposing the pt to become more acidemic. Renal 21 Thur 03/20/03 9am Dr. Mallet Le Giang Proscribe Samera Kasim Page 3 of 7 iii. Bicarb reabsorption will decrease because H+ secretion is required to capture the filtered bicarb iv. A patient with alkylemia and edema, the CA inhibitory would be an ideal diuretic e. Na reabsorption: more Na reabsorption = more H+ secretion. i. The entry of one cation favors the entry of another to maintain neutrality ii. Diuretics decrease Na reabsorption in the proximal tubule. Thus there is a lot of Na in the lumen upon arriving at the collecting duct. That increases Na reabsorption there, resulting in increased K secretion and H+ secretions iii. Diuretics can predispose a person to developing alkylemia. f. Change in extracellular pH i. K+ can influence H+ secretion; they generally move in opposite directions ii. If you decrease the extracellular K+ conc, K leaves the cell down its cg and H+ enters iii. H+ intracellular conc and secretions increase iv. Hypokalemia leads to increased H+ secretion g. Aldosterone: i. Stimulates Na reabsorption and K and H+ secretion ii. Activates H+ ATPases in the luminal membrane, directly stimulating H+ secretion iii. **Conn’s disease (hyperaldosteronism) leads to alkylemia (because too much H+ is being excreted) and hypokalemia iv. **Addision’s diseases is opposite Conn’s VII. Diuretic abuse a. Again, can cause alkalemia b. causes K+ depletion (by secretion) c. this favors H+ secretion d. causes extracellular volume to become depleted: decreased Blood pressure e. Incr. renin, Angiotensin and Aldosterone secretions f. Aldosterone Stimulates H+ secretion directly and via K+ depletion VIII. Disturbances in acid-base balance a. Normal arterial plasma pH range: 7.35-7.45 b. Definitions: c. Acidemia: a reduction in arterial pH below 7.35 d. Acidosis: any abnormal condition that produces acidemia e. Alkalemia: an increase in arterial pH above 7.45 f. Alkalosis: any abnormal condition that produces alkalemia IX. Respiratory acid-base disturbances Renal 21 Thur 03/20/03 9am Dr. Mallet Le Giang Proscribe Samera Kasim Page 4 of 7 a. Caused by change in PCO2 b. Respiratory acidosis: increased arterial PCO2 ↑ CO2 + H2O ↔ ↓H+ + ↑ HCO3i. If the lungs are not eliminating CO2 at the same rate of metabolic metabolism (pulmonary insufficiency, emphysema), levels rise and the above reaction occurs ii. The pH falls even though bicarb is being produced because pH specifically refers to the H+ concentration; it is independent of HCO3 by definition. iii. iv. Renal response: increased H+ secretion restores extracellular pH by up-regulating the production of ammonium, resulting in further increases in HCO3c. Respiratory alkalosis: decreased arterial PCO2 ↓ CO2 + H2O ↔ ↑H+ + ↓HCO3i. Occurs in hyperventilation ii. Renal response: less H+ secretion, more HCO3-excretion in urine d. The changes in bicarb may seem counterintuitive. X. Metabolic acidosis a. NOT because of problems with the lungs b. Low plasma pH due to: i. gain of fixed acid in body fluids, or ii. loss of HCO3iii. In either case, [HCO3-] falls 1. remember bicarb incr in RESPIRATORY acidosis c. Renal compensation: increased H+ secretion (more ammonium in urine); production of new HCO3d. Respiratory compensation: increased ventilation (peripheral chemoreceptors stimulated), decreases PCO2 which eliminates a source of H+ from the body e. Metabolic acidosis can be causes by diarrhea. Why? i. Bicarb is being lost from the GI tract. ii. As bicarb conc falls, the equilibrium is shifted right iii. More H+ ions are made = acidosis. f. Lactic acid from anaerobic metabolism (indicative of a hypoxic environment) breaks down to lactate and H+. g. Bicarb tries to neutralize the excess H+, resulting in a decrease in bicarb concentration XI. Metabolic alkalosis a. Abnormally high plasma pH, due to i. Excessive gain of strong base or HCO3ii. Excessive loss of fixed acid Renal 21 Thur 03/20/03 9am Dr. Mallet Le Giang Proscribe Samera Kasim Page 5 of 7 b. HCO3- concentration rises due to shift in carbonic anhydrase equilibrium toward HCO3c. Respiratory compensation: decreased ventilation (increases PCO2) d. Renal compensation: i. Incomplete reabsorption of filtered HCO3- (which spills into urine) ii. β-intercalated cells secrete HCO3e. vomiting increases bicarb because acid is being lost; Resp decreases XII. Davenport diagram depicts changes in acid-base metabolites a. The graph will not be on the exam, but it’s important that you understand the concepts of the carbonic anhydrase reaction. b. “A” is indicative of the normal bicarb concentration and pH of extracellular fluid. c. EX: Respiratory acidosis and renal compensaton: increasing PCO2; If you move Left along the horizontal axis, indicating a drop in the pH, the bicarb concentration increases along the vertical axis to restore the normal pH of 7.4 by response of the kidneys. More ammonium will be in the urine. d. The opposite is true for an increase in the pH (bicarb conc decr) e. Understand these concepts for both metabolic and respiratory acid-base imbalances with compensation f. Renal failure: results in acidosis. kidneys should compensate to the acidosis and results in increased ammonium in the urine. g. the lungs should compensate as well: an increase in ventilation by stimulation of peripheral chemoreceptors will decrease the PCO2, and elimination of H+ from the body. h. There will be a scenario on the Exam for Acid-Base disorders. Recognize whether compensations are occurring for the given lab values. Be able to recognize what kind of acid-base disorder the pt has. XIII. Anion gap a. Used in differential diagnosis of metabolic acidosis b. A.G. = measured cation (Na+) - measured anions (Cl-, HCO3-) c. Normal range: 5-12 mEq/l i. Plasma proteins are anionic but are not measured in the lab, thus the [Na] should be greater. There is normally only a 5-12 mEq/l difference, which is the normal range. d. Anion gap is either normal or increased, depending on cause of metabolic acidosis e. Hyperchloremic acidosis: A.G. is unchanged HCl + HCO3- Cl- + H2O + CO2 i. Loss of HCO3- is matched by gain of Clii. The increase in urine Cl- is excreted in the urine Renal 21 Thur 03/20/03 9am Dr. Mallet Le Giang Proscribe Samera Kasim Page 6 of 7 iii. Seen in diarrhea: kidneys respond to the loss of volume by retaining Cl- and Na+. over time there is an incr in Cl- because less is being excreted in the urine. Cl- will replace the bicarb that was lost (both anions). f. High anion gap acidos is: HCO3- is replaced by unmeasured anion: HA + HCO3- A- + H2O + CO2 i. (“unmeasured” means that the lab doesn’t measure that anion) ii. In this case, anion gap increases iii. Bicarb is consumed to neutralize free H+ g. Causes of high anion gap acidosis: i. You can find the anion gap in E. Elm Park: ii. Ethanol iii. Ethylene glycol iv. Lactic acid v. Methanol vi. Paraldehyde vii. Aspirin viii. Renal failure ix. Ketone bodies XIV. Case: ethylene glycol intoxication a. A 55 y.o. alcoholic man is found unconscious and taken to the ER by the police. An open container of antifreeze is found next to the patient. On physical exam, his blood pressure is 110/70, pulse 80/min, and respiratory rate 30 breaths/min. Arterial plasma chemistries: Normal Na+, mEq/l 125 135-145 Cl-, mEq/l 80 96-106 K+, mEq/l 4.0 3.5-4.5 HCO3-, mEq/l 15 23-29 pH 7.20 7.35-7.45 XV. What is patient’s anion gap? Why is respiratory frequency so high? Why is HCO3so low? Will arterial PCO2 be above or below normal? a. Anion gap = 125 – (80 + 15) = 30 b. Low bicarb because it’s buffering the excess H+ from the ethylene glycol c. PCO2 will be above normal i. He has acidosis ii. Has increases respiratory rate d. Gap metabolic acidosis XVI. A bad case of the flu a. A 40 y.o. woman presents with a 7 day history of fever, chills, and severe vomiting. Physical exam: unsteady ambulation, weakness, poor skin Renal 21 Thur 03/20/03 9am Dr. Mallet Le Giang Proscribe Samera Kasim Page 7 of 7 turgor, dry mucous membranes. Supine blood pressure 130/70, pulse 100; upon standing, 105/60 and 120. Arterial plasma chemistries: Normal + Na , mEq/l 142 135-145 Cl-, mEq/l 100 96-106 HCO3 , mEq/l 36 23-29 b. c. d. e. This pt is hypovolemic Has orthostatic hypotension and tachycardia Na and water are lost in proportion, thus Na levels look normal Predict the changes in arterial plasma pH and PCO2 in this patient compared to normal. i. Vomiting results in the loss of acid, thus she’s alkalotic ii. Also, extracellular volume is low, thus ADH, Angiotensin and aldosterone are high. Aldosterone stimulates the secreation of H+ in the collecting ducts, further adding to the alkalosis. f. Why is HCO3- so high? i. Loss of acid during vomiting produces alkalosis g. Is anion gap increased? i. No, it’s small (6mEq/l) h. Is her plasma K+ likely to be normal? i. No, has hypokalemia ii. Results in increased H+ secretion XVII. SUMMARY: Session 9 a. Six factors control renal H+ secretion: Intracellular pH, plasma PCO2, carbonic anhydrase, Na+ reabsorption, EC K+, aldosterone b. Cellular defense mechanisms against pH changes c. Respiratory acid-base disturbances: primary changes in PCO2 cause H+ and HCO3- to change ( secondary change) d. Metabolic acid-base disturbances: gains or losses of H+ and HCO3-; respiratory, renal responses i. Respiration is secondary change to PCO2 e. Anion gap: differential diagnosis of metabolic acidosis