Microsoft Word
advertisement
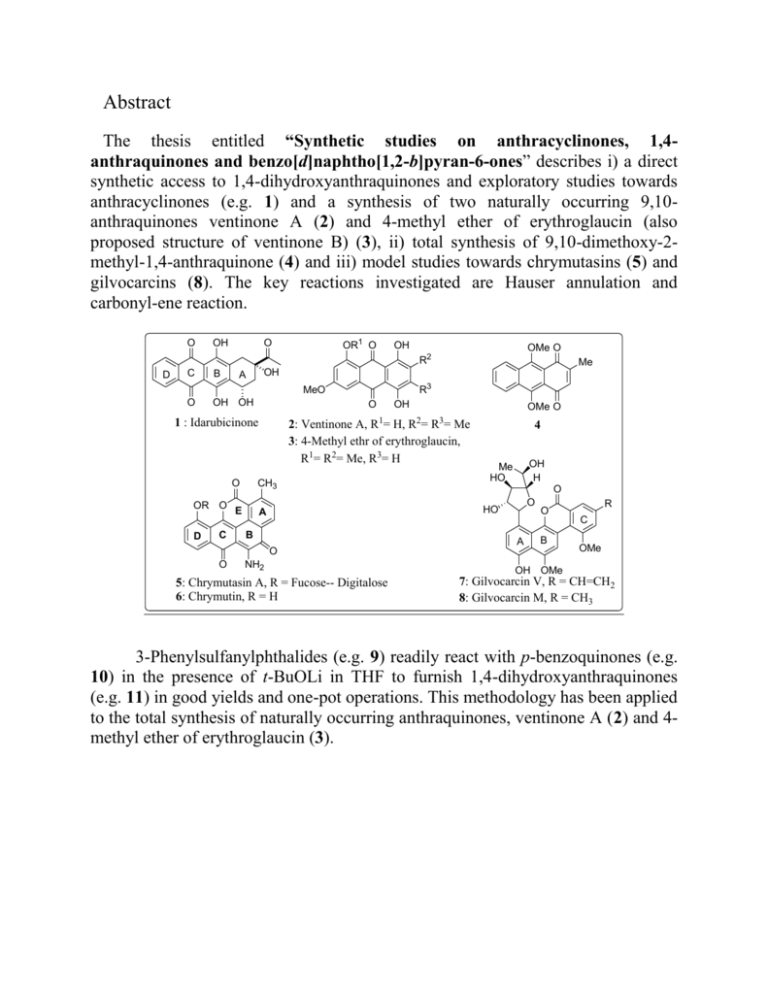
Abstract The thesis entitled “Synthetic studies on anthracyclinones, 1,4anthraquinones and benzo[d]naphtho[1,2-b]pyran-6-ones” describes i) a direct synthetic access to 1,4-dihydroxyanthraquinones and exploratory studies towards anthracyclinones (e.g. 1) and a synthesis of two naturally occurring 9,10anthraquinones ventinone A (2) and 4-methyl ether of erythroglaucin (also proposed structure of ventinone B) (3), ii) total synthesis of 9,10-dimethoxy-2methyl-1,4-anthraquinone (4) and iii) model studies towards chrymutasins (5) and gilvocarcins (8). The key reactions investigated are Hauser annulation and carbonyl-ene reaction. O OH OR1 O O OH OMe O R2 D C B O OH OH R3 MeO O OR O D C OH 1 1 : Idarubicinone O OMe O 2 3 2: Ventinone A, R = H, R = R = Me 3: 4-Methyl ethr of erythroglaucin, R1= R2= Me, R3= H CH3 E Me OH A 4 OH H Me HO O O HO A B A O B O O NH2 5: Chrymutasin A, R = Fucose-- Digitalose 6: Chrymutin, R = H R C OMe OH OMe 7: Gilvocarcin V, R = CH=CH2 8: Gilvocarcin M, R = CH3 3-Phenylsulfanylphthalides (e.g. 9) readily react with p-benzoquinones (e.g. 10) in the presence of t-BuOLi in THF to furnish 1,4-dihydroxyanthraquinones (e.g. 11) in good yields and one-pot operations. This methodology has been applied to the total synthesis of naturally occurring anthraquinones, ventinone A (2) and 4methyl ether of erythroglaucin (3). SPh O O R1 t-BuOLi, R2 60 oC to r.t. R1 X O X OH R2 O O OH O 30-70% 11 10 R1, R2 = alkyl, O-alkyl, -(CH2)4-, -(CH2)3CH(OH)-, -CH=CH-CH=CH-, etc. 15 examples have been worked out 9 Intramolecular carbonyl-ene reaction of 2-methallylated anthraquinone aldehyde 12, obtained in 10 steps has been effected for the synthesis of 13, core structure of idarubicin. Modest attempts have been made to extend the carbonyl-ene reaction, for the first time, to the synthesis of substituted naphthalene 16 and phenanthrene 17 as illustrated below. O OH SnCl4 . 5H2O dry DCM CHO O OMe OH OH 13 CHO O O OH OH 1 SnCl4 . 5H2O dry DCM 83% 83% OMe 15 OMe 14 OH OH O OH SnCl4 . 5H2O CHO dry DCM O OH 89% 12 OMe O 16 17 The first yet short synthesis of the unusual anthraquinone 4 has been achieved in 5 steps from p-benzoquinone using Kochi-Anderson radical methylation as a key step. During the course of the synthesis few unprecedented cyclopropaanthraquinones like 19, 20 and 21 have been synthesized and their chemistry studied. OMe O OMe O 18 AgNO3, AcOH, (NH4)2S2O8, CH3CN-H2O reflux OMe O OMe O 4 (42%) O SPh + K2CO3, Me2SO4 O O 19 OMe O OH O t-BuOLi, THF O – 60 C 48% acetone, reflux 65% OH O OMe O 21 20 9 Cyanophthalide 22 has been shown to undergo a tandem annulation with methyl 5,5-dimethoxy-2-methyl-6-oxo-5,6-dihydronaphthalene 1-carboxylate (23), obtained in 10 steps from 6-methoxy-1-tetralone, to fabricate a chrymutasin chromophore 5 in one-pot operation and thus O-Methylhayumicinone (25) has been synthesized during this investigation. Both C and E rings are formed in one operation. CH3 MeO2C OMe CN t-BuOLi O+ O O 23 22 OMe THF - 60 °C OMe 78% O CH3 E A OMe O D C D C O CH3 10% HCl, OR O MeOH-H2O E OMe 70% B OH O O OR O A B O OMe O OH 25 R = Me 24 CH3 O O NH2 5 R = Sugar The tandem strategy has been applied to the regiospecific construction of the core structure of gilvocarcin chromophore (e.g. 26) from O-benzylcyanophthalide (27), prepared in 9 steps, and styryl sulfone 28, prepared in 6 steps. But, the total synthesis of defucogilvocarcin M (32) has been foiled, due to the failures in desulfonylation of 26. O CN MeO2C Me t-BuOLi, THF O OBn O 27 PhO2S OMe - 60 oC 10-15% OBn O 26 28 9 steps O 6 steps Me O OHC Me CO2Et O Me Me + 29 30 OMe H2N OH 31 OH OMe 32 Me O OMe SO2Ph Key words: Synthesis, anthracyclinone, 1,4-anthraquinone, cyclopropaanthraquinone, benzonaphthopyranone, Hauser annulation, intramolecular carbonyl-ene reaction, Kochi-Anderson radical alkylation reaction, Claisen rearrangement, Heck reaction. 3-Phenylsulfanylphthalides (e.g. 8a) readily react with p-benzoquinones in the presence of LiOtBu in THF to furnish 1,4-dihydroxyanthraquinones in good yields and one-pot operations. A Hauser-initiated tandem annulation has been developed for the rapid regiospecific synthesis of benzonaphthopyranones. This strategy has been generalized with benzonaphthopyranones 26, 29, 32 & 35. It has also been employed in a short synthesis of chartarin (3) and Omethylhayumicinone (67). The synthesis of 9,10-dimethoxy-2-methyl-1,4-anthraquinone, an unusual quinone, is achieved in 5 steps from p-benzoquinone. A Kochi-Anderson radical methylation features as a key step in the synthesis. The chemistry of a cyclopropa-1,4-anthracenedione is also described.