Case Study 1
advertisement

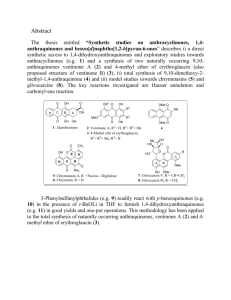
Case Study 1: Catalytic Hydrogenation of Enamides The Nobel Prize for 2002 was awarded to Noyori, Knowles, and Sharpless for their pioneering work in asymmetric catalysis. This case study focuses on the techniques utilized to unravel the mechanism of asymmetric hydrogenation of prochiral enamides. Useful References: Knowles, W. S. Acc. Chem. Res. 1983, 16, 106-112. Halpern, J. Science 1982, 217, 401. Halpern, J.; Riley, D. P.; Chan, A. S. C.; Pluth, J. J. Am. Chem. Soc. 1977, 99, 8055. Chan, A. S. C.; Pluth, J. J.; Halpern, J. Inorg. Chim. Acta 1979, 37, L477. Chan, A. S. C.; Halpern, J. J. Am. Chem. Soc. 1980, 102, 838. Chan, A. S. C.; Pluth, J. J.; Halpern, J. J. Am. Chem. Soc. 1980, 102, 5952. Landis, C. R.; Halpern, J. J. Am. Chem. Soc. 1987, 109, 1746-1754. In 1975 Knowles and coworkers reported that in situ reaction of [Rh(DIPAMP)(COD]+BF4- with acetamidocinnamic acid in alcohol solutions and under an H2 atmosphere led to rapid hydrogenation of the C=C bond with 95% enantioselectivity. MeO2C H N [Rh(DIPAMP)(COD)]+ O Ph MAC + H2 (3.5-27atm) MeO2C H N O Ph (R-S)/(R+S)=95.7% enantiomeric excess O P P DIPAMP O Substrates: Good ee if they can chelate and have an electron withdrawing group in position. Interesting observation: e.e. was lowered by higher pressure of H2 but this effect could be offset by using higher temperature. Fundamental Mechanistic Issues 1. 2. 3. 4. Hydride Route or Unsaturate Route? Rate Controlling Step? Selectivity Controlling Step? Origin of Enantioselection? Mechanistic Starting Point: Simple Achiral Model Complexes P P DIPHOS Strategy: 1. Study reaction of catalyst precursor with H2 2. Study reaction of catalyst precursor with substrate 3. Measure alkene binding constants and rates 4. Determine rate law of catalytic reaction. 1. Reactions with H2 Ph Ph P Rh P Ph Ph yellow + H2 methanol solution orange 31 P NMR: 60, JRh-P 160Hz 31 P NMR: d 60, JRh-P 203 Hz Gas Uptake: 2 H2 /Rh 1 H NMR: norbornane, no Rh-H (<0 ppm) contrasts with Ph Ph Ph P Ph P Rh Ph Ph orange colorless + H2 methanol solution 31 P NMR: d 47.2, JRh-P 121 Hz Gas Uptake: 3 H2 /Rh 1 H NMR: norbornane, -12.4, JRh-H =23Hz, JP-H=15Hz These data are consistent with the products shown below: Ph Ph H OMe P Rh P OMe H Ph Ph Ph Ph H H OMe P Ph Rh Ph P H Ph MeO Ph H Apparently the trans influence of H is large enough to preclude formation of a dihydride from the reaction of H2 with [(DIPHOS)Rh(norbornadiene)]+. Crystals of the product formed from H2 and [(DIPHOS)Rh(norbornadiene)]+ revealed an unusual dimeric structure shown below: Ph PPh2 P 2+ Rh Rh Ph2 P P Ph 2. Binding of Unsaturates A number of unsaturates bind to the di-solvate, 1. Ph Ph H OMe P Rh + P OMe H Ph Ph 1 Ph Ph P Rh P Ph Ph 2 This can be monitored by NMR or by changes in the visible spectrum. The unsaturates do not absorb in the visible region whereas 1 and 2 do. Beginning with a solution of 1 before the addition of benzene: Abs0=1[1]0 initial absorbance After addition of some benzene Abs=1[1] + 2[2] Often the value of 2 is not known. The equilibrium constant is given by: [2] K [1][benzene] [2] [1]0 [2] [benzene]0 [2] solve for [2] -([1]0 [benzene]0 K 1) ([1]0 [benzene]0 K 1)2 4[benze [2] = 2 The concentration of 1 at any point in the titration is given by [1] [1]0 [2] Abs 1 [1]0 [2] 2 [2] Substitution of the expression for [2] into this equation leads to a description of the absorbance in terms of known values ([1]0, 1, and [benzene]0) and unknowns (K and 1). Nonlinear least squares analysis allows one to fit the unkowns to the observed absorbance data. Using this method the following data are obtained for binding different unsaturates. Ph Ph H OMe P Rh + P OMe H Ph Ph 1 Unsaturate Benzene Toluene p-Xylene Methyl acrylate 1-Hexene Styrene O OMe H N MAC O Ph Ph Ph P Rh P Ph Ph 2 K (M-1) 18 97 500 3 2 20 5.3x103 The binding of simple alkenes is weak; only one alkene binds to the Rh center as indicated by the inequivalent phosphines ligands in the 31P NMR spectrum: Ph Ph H OMe P Rh + P OMe H Ph Ph 1 Ph Ph P Rh P Ph Ph H OMe 3. Measurement of the Kinetics of Approach to Equilibrium A) One approach to measure the binding rates is stopped-flow measurement of absorbance time profiles accumulated upon addition of excess MAC to 1. Ph Ph H OMe P Rh + MAC P OMe H Ph Ph 1 k1 k-1 Ph Ph O P Rh NH P MeO Ph Ph Ph O According to 1st-Order Approach to Equilibrium Kinetics k1 [MAC]k1 t [A]t [A] ([A]0 [A] )e using non-linear least squares analysis one can optimize [A], [A]0, and kobs = k1[MAC]+k-1. From a series of experiments at different [MAC] one obtains kobs MAC Analysis of k1[MAC] + k-1 as a function of MAC concentration gives well-determined values of k1 but not k-1. The value of k1[MAC] is so large that the value of k-1 is subsumed. k1=2.7x104M-1s-1 B) The rate of MAC dissociation can be determined from K and k1. Independent verification of this rate comes from the kinetics of displacement of MAC by arenes. Trapping Approach