This request is for: A - Prescribing of an Unlicensed Medicine
advertisement

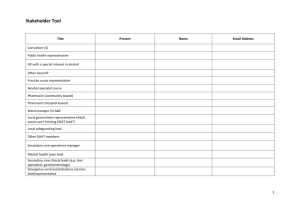
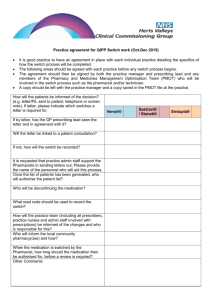
NHS FORTH VALLEY UNLICENSED MEDICINE TREATMENT REQUEST How to complete this form: This form should be completed by the requesting clinician/ clinical pharmacist where a medicine that does not contain a UK product licence is being considered for use OR is an Expanded Access Medication. All sections of the form must be completed, and agreement to prescribe obtained prior to prescribing/requesting the medicine to ensure that delays in treatment are minimised. What to do with the form once complete: The requesting consultant should send the completed form to Medicines Information, Pharmacy Department. Where the treatment cost ≥ £10,000 per patient per annum or treatment cost for estimated total number of patients exceeds £50,000, the form will be forwarded to the Exceptions Committee for consideration. SECTION 1: PRESCRIBER, PATIENT & MEDICINE DETAILS Patient Details: Attach addressograph or use patient CHI number and postcode CHI Number: Ward/ department/ practice: Postcode: Name of Prescriber: (print clearly in capitals) Contact number: Medicine name and formulation requested Indication: Version 1 23rd March 2011 UNCONTROLLED WHEN PRINTED SECTION 2a Details of the Unlicensed Medicine (ULM) and the Clinical Evidence supporting this request. (To be completed by the clinical pharmacist/the prescriber) This request is for: Please tick box A - Prescribing of an Unlicensed Medicine B - Prescribing of a medicine from a completed Clinical Trial Product Name:………………………………………………….................…………………………………. Proprietary Name (if applicable)……………………………………………………………..:……..……… Pharmaceutical Form: ………………………………………………………………………………………. Strength: ……………………………….………………….………………………………………………….. Manufacturer: (if known)…………...……………………….……………………………………………….. Dose and frequency: ……………………………..……………..…………………………………………… Route: ………………………………………..…….…………………………………………………………. . Length of treatment:………………………………………..………………………………………………… Cost of each treatment course per patient:……………………………………………………………….. Estimated number of patients:……………………………………………………………………………… Section 2b Clinical Evidence supporting this request A practitioner prescribing an unlicensed medicine or a licensed medicine for an unlicensed condition does so under his/her own responsibility. Consequently, he/she carries the burden of the patient’s welfare and may be called upon to justify their actions. A pharmacist will share the responsibility in situations where they have been involved in the procurement and purchase of an unlicensed medicine, where this involves specifying the product or if their actions/omissions have contributed to harm. Pharmacists will not be held accountable for “off label” use. Clinicians must make patients aware of the unlicensed nature of the treatment and gain their consent before use. This should be documented in the patient’s clinical notes. Version 1.00 Page 2 of 4 UNCONTROLLED WHEN PRINTED It is the responsibility of the requesting clinician to demonstrate the clinical case for the individual patient, such that the following criteria are satisfied: • demonstrate that the patient’s potential response to treatment with the unlicensed medicine outweighs the risk of using an unlicensed product. and • clearly describe the arrangements to monitor and review the patient’s response to the medicine. Is there any clinical evidence for prescribing this medicine? Y/N (delete as appropriate) (Please attach evidence to this form) What are the risks to the patient not receiving this medicine? ……………………………………. What special precautions / monitoring is required whilst the patient is receiving this medicine? …………………………………………………………………………………………………….. What are the reported side effects / toxicity of this medicine? ……………………………………… SECTION 3 Final Approval Process (To be completed by the requesting Consultant/ Clinical Pharmacist and AMD.) Name of Consultant: Name of Clinical Pharmacist: ………………………….…………… ……. …………………………………… ……. Signature of Consultant: Signature of Clinical Pharmacist: ……………………………………… …….. …………………………………… ……. Unit/Speciality ……………………………….……… ……. Date: ………………………. Date: …………………………. Version 1.00 Page 3 of 4 UNCONTROLLED WHEN PRINTED Name of Associate Medical Director ………………………… Signature of Associate Medical Director ………… … … … … … … … … … … Date … … … NOTE: For treatments costing < £10,000 per patient per annum, the AMD is responsible for alerting the General Manager and Finance if the cost or the demand for use will have significant impact on budgeting. For treatments costing ≥ £10,000 per patient per annum or >£50,000 per annum for the total potential number of patients, the AMD is responsible for considering the use on clinical grounds but the request must be forwarded to the Exceptions Panel for final approval before prescribing. In this instance the completed form must be sent to the Medicines Information Pharmacist for processing through the exceptions pathway. In some cases the AMD may wish to refer approval of the use of this medicine to the Chair of the Drug and Therapeutics Committee. PLEASE FORWARD THE COMPLETED FORM TO -The Medicines Information Pharmacist. Version 1.00 Page 4 of 4 UNCONTROLLED WHEN PRINTED