GP Agreement (Sastravi, Stanek, Sirdupla
advertisement
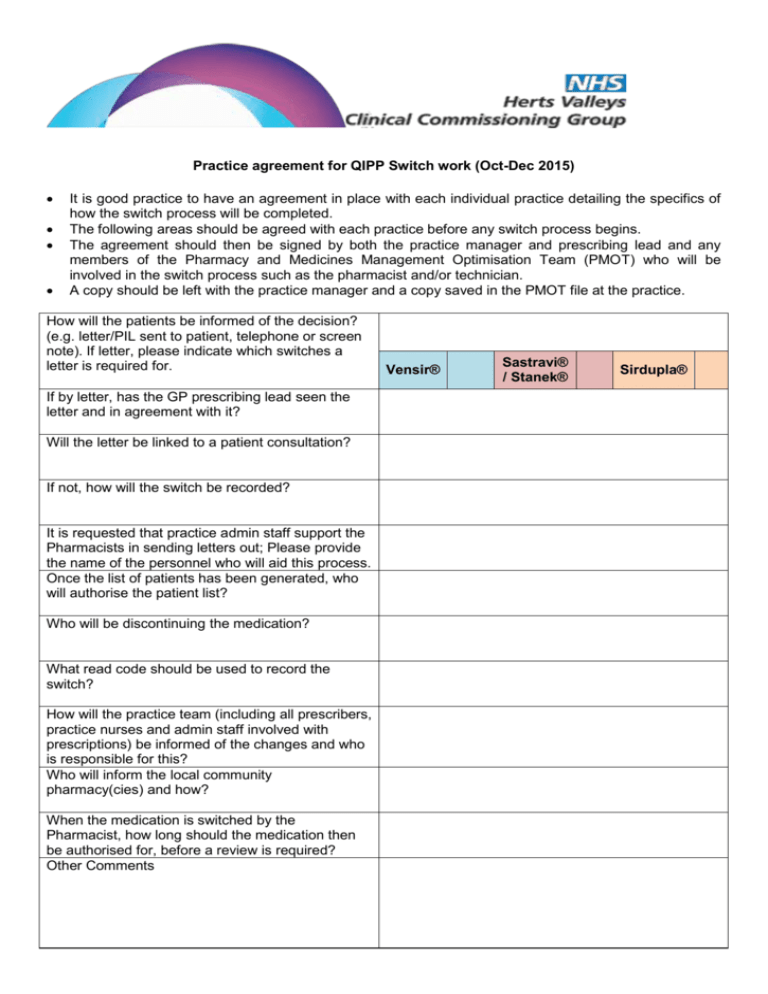
Practice agreement for QIPP Switch work (Oct-Dec 2015) It is good practice to have an agreement in place with each individual practice detailing the specifics of how the switch process will be completed. The following areas should be agreed with each practice before any switch process begins. The agreement should then be signed by both the practice manager and prescribing lead and any members of the Pharmacy and Medicines Management Optimisation Team (PMOT) who will be involved in the switch process such as the pharmacist and/or technician. A copy should be left with the practice manager and a copy saved in the PMOT file at the practice. How will the patients be informed of the decision? (e.g. letter/PIL sent to patient, telephone or screen note). If letter, please indicate which switches a letter is required for. If by letter, has the GP prescribing lead seen the letter and in agreement with it? Will the letter be linked to a patient consultation? If not, how will the switch be recorded? It is requested that practice admin staff support the Pharmacists in sending letters out; Please provide the name of the personnel who will aid this process. Once the list of patients has been generated, who will authorise the patient list? Who will be discontinuing the medication? What read code should be used to record the switch? How will the practice team (including all prescribers, practice nurses and admin staff involved with prescriptions) be informed of the changes and who is responsible for this? Who will inform the local community pharmacy(cies) and how? When the medication is switched by the Pharmacist, how long should the medication then be authorised for, before a review is required? Other Comments Vensir® Sastravi® / Stanek® Sirdupla® Venlafaxine MR 75mg/150mg caps/tabs (branded and generics) TO Vensir XL 75mg/150mg capsules Switch process approved by: Practice Manager Practice GP Prescribing Lead PMOT Pharmaceutical Adviser Date Stalevo® all strengths (branded and generics) TO Stanek tablets OR Sastravi tablets Sastravi® tablets Current choice of WHHT Contains Soya oil (hence allergy status for patients needs to be thoroughly checked) Stanek® tablets Community Pharmacist’s loose out financially by dispensing this brand Does NOT contain any soya oil Two options are available for the Stalevo® switch. Both are the same price. Please tick which option you wish to opt to switch to. Switch process approved by: Practice Manager Practice GP Prescribing Lead PMOT Pharmaceutical Adviser Date Seretide Evohaler 125mcg/250mcg (branded and generics) TO Sirdupla MDI 125mcg/250mcg for adult patients with asthma (18 years and above) YES NO Is the practice happy for Seretide evohaler (125mcg and 250mcg) prescribed for COPD (unlicensed indication) to be switched to Sirdupla MDI (also unlicensed indication) Switch process approved by: Practice Manager Practice GP Prescribing Lead PMOT Pharmaceutical Adviser Date I, the CCG Pharmacist/Pharmacy Technician, hereby agree to comply with the Data Protection Act 1998 and to ensure that patient confidentiality is maintained at all times with a duty of care equivalent to that required by the GPhC standards of conduct, ethics and performance. Name Signature Date










