CHEM 121 Spring 2008 Test State Comp. Lab Science 4b
advertisement
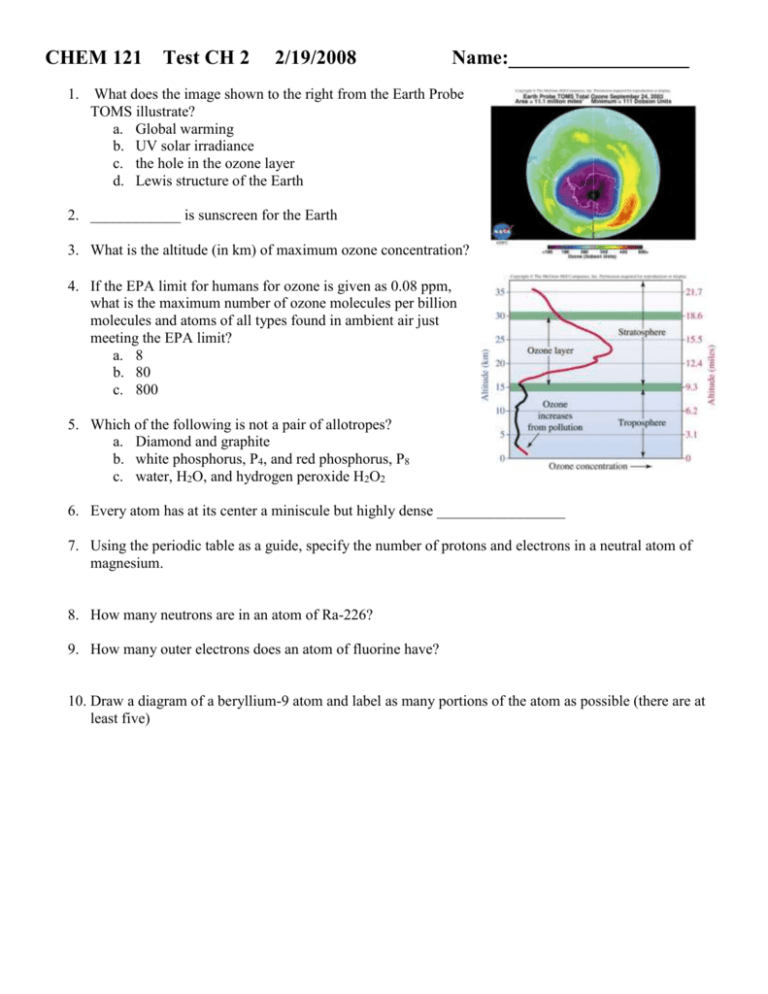
CHEM 121 1. Test CH 2 2/19/2008 Name:__________________ What does the image shown to the right from the Earth Probe TOMS illustrate? a. Global warming b. UV solar irradiance c. the hole in the ozone layer d. Lewis structure of the Earth 2. ____________ is sunscreen for the Earth 3. What is the altitude (in km) of maximum ozone concentration? 4. If the EPA limit for humans for ozone is given as 0.08 ppm, what is the maximum number of ozone molecules per billion molecules and atoms of all types found in ambient air just meeting the EPA limit? a. 8 b. 80 c. 800 5. Which of the following is not a pair of allotropes? a. Diamond and graphite b. white phosphorus, P4, and red phosphorus, P8 c. water, H2O, and hydrogen peroxide H2O2 6. Every atom has at its center a miniscule but highly dense _________________ 7. Using the periodic table as a guide, specify the number of protons and electrons in a neutral atom of magnesium. 8. How many neutrons are in an atom of Ra-226? 9. How many outer electrons does an atom of fluorine have? 10. Draw a diagram of a beryllium-9 atom and label as many portions of the atom as possible (there are at least five) 11. Give the symbol showing the atomic number and the mass number for the element that has 26 protons and 30 neutrons. 12. Draw the Lewis structure for CCl2F2. Carbon is the center atom. 13. Draw the Lewis structure for HCN. Carbon is the center atom. 14. Draw the two resonance forms for ozone, O3. 15. 1 nm = a. 10-6 m b. 10-7 m c. 10-9 m 16. An orange photon has a wavelength of 640 nm. What is this wavelength in the measurement unit of meters? a. 6.4 x 10-7 m b. 6.4 x 10-9 m c. 6.4 x 10-5 m 17. The wavelength is the _______________ between successive peaks. 18. Draw the electromagnetic spectrum including large arrows that indicate how energy, wavelength, and frequency change. 19. The _________________ is the number of waves passing a fixed point in one second. 20. Consider these four types of radiant energy from the electromagnetic spectrum: infrared, microwave, ultraviolet, visible a. Which of these has the longest wavelength? ________________ b. Which of these has the highest energy?___________________ 21. A UV photon has a wavelength of 280 nm. What is the frequency of this UV photon? 22. _____________ are individual bundles of energy 23. calculate the energy of a photon of light having a frequency of 2.3x107 s-1. 24. Why is UV light harmful to the skin of humans and visible light is not? a. UV radiation is of sufficient energy to cause molecular bonds to break b. UV radiation is better absorbed by the skin c. Skin has a natural sunscreen against visible light 25. Which chemicals in the atmosphere absorb UV-C? 26. Which category of UV radiation is of the greatest concern to humans on the surface of the Earth (you should consider both the energy of the UV radiation as well as the atmospheric interaction)? a. UV-A b. UV-B c. UV-C 27. Which of the following UV-B photons would be more harmful to humans (disregard atmosphere)? a. 285 nm b. 286 nm c. 287 nm 28. Concerning the biological effects of ultraviolet radiation, which of the following is incorrect? a. the consequences depend on the sensitivity of the organism to that radiation b. the consequences depend on the Lewis structure of the radiation c. the consequences depend on the energy associated with the radiation 29. Which area of the United States has the highest solar ultraviolet irradiance in kilojoules per square mile? a. The area in a 100 mile radius around Silver city b. Utah c. Florida 30. How are chlorine radicals produced from CFCs? a. UV radiation breaks a carbon-halogen bond b. the CFCs react with oxygen c. the CFCs combined with smog to form chlorine radicals 31. Why are chlorine radicals so effective at destroying ozone molecules? a. Because they are highly reactive b. because they act as a catalyst c. because they are in high concentrations 32. What three elements are chlorofluorocarbons made of? 33. The EPA has used the slogan "ozone: good up high, bad nearby." Explain how ozone could be both good and bad. 34. Chemical formulas for individual CFCs, such as CFC-11 (CCl3F), can be figured out from their code numbers. One way to interpret the code number for CFCs is to add 90 to the number. In this case 90 + 11 = 101. The first number in the sum is the number of carbon atoms, the second is the number of hydrogen atoms, and the third is the number of fluorine atoms. CCl3F has one carbon, no hydrogen, and one fluorine atom. All remaining bonds are assumed to be chlorine until carbon has the required four single covalent bonds to satisfy the octet rule. Another example is CCl4 which has a code number of CFC-10. What is the code number for CCl2F2? a. CFC-10 b. CFC-11 c. CFC-12 d. CFC-13 e. CFC-14 35. My study strategy for this class is… Useful Constants h = 6.626*10-34 Js









