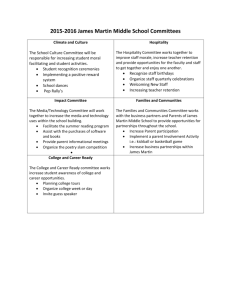
Publications - King`s College London
advertisement

Gary P. Martin Publications (1) Authored Book. Zeng, X.M., Martin, G.P. and Marriott, C. Particulate interaction in dry powder formulation for inhalation. (ISBN 0-7484-0960-2) Taylor & Francis, London, 2001. (2) Refereed Papers in Scientific Journals. 1. Martin, G.P., Marriott, C. and Kellaway, I.W. Direct effect of bile salts and phospholipids on the physical properties of mucus. Gut, 19, 103-107, 1978. 2. Martin, G.P., Kellaway, I.W. and Marriott, C. The solubilisation of progesterone by mixed bile salt phospholipid sols. Chem. Phys. Lipids, 22, 227-238, 1978. (http://dx.doi.org/10.1016/0009-3084(78)90029-4). 3. Martin, G.P., Marriott, C. and Kellaway, I.W. The interaction of progesterone with mucus glycoproteins. Pharm. Acta Helv., 56, 5-8, 1981. 4. Martin, G.P. Invited commentary on the role of bile acid reflux in acute haemorrhagic gastritis. World J. Surg., 5, 197-198, 1981. (http://dx.doi.org/10.1007/BF01658288). 5. Martin, G.P. and Marriott, C. Membrane damage by bile salts: the protective function of phospholipids. J. Pharm. Pharmacol., 33, 754-759, 1981. (http://dx.doi.org/ 10.1111/j.2042-7158.1981.tb13926.x). 6. Turner, N.C., Martin, G.P. and Marriott, C. The influence of native porcine gastric mucus gel on hydrogen ion diffusion: the effect of potentially ulcerogenic agents. J. Pharm. Pharmacol., 37, 776-780, 1985. (http://dx.doi.org/10.1111/j.2042-7158.1985.tb04967.x). 7. Martin, G.P., Newbery, R.S., Turner, N.C. and Marriott, C. Gastric mucosal injury by bile acids: the influence of phospholipids. Gastroenterol. Clin. Biol., 9, 80-83, 1985. 8. Topham, J.D. and Martin, G.P. The determination of yield values using a BP plate plastometer. J. Pharm. Pharmacol., 37, 847-848, 1985. (http://dx.doi.org/10.1111/j.2042-7158.1985.tb04987.x). 9. Queen, D., Martin, G.P., Marriott, C. and Fairbrother, J.E. Assessment of the potential of a new hydrocolloid dermatological patch (Actiderm) in the treatment of steroid-responsive dermatoses. Int. J. Pharm., 44, 25-30, 1988. (http://dx.doi.org/10.1016/0378-5173(88)90096-8). 10. Martin, G.P., Bell, A.E. and Marriott, C. An in vitro method for assessing particle deposition from metered pressurised aerosols and dry powder inhalers. Int. J. Pharm., 44, 57-63, 1988. (http://dx.doi.org/10.1016/0378-5173(88)90100-7). 11. Jacobs, M., Martin, G.P. and Marriott, C. Effects of phosphatidylcholine on the topical bioavailability of corticosteroids assessed by the human skin blanching assay. J. Pharm. Pharmacol., 40, 829-833, 1988. (http://dx.doi.org/10.1111/j.2042-7158.1988.tb06283.x). 12. Wilkins, K.M., Hanlon, G.W., Martin, G.P. and Marriott, C. The migration of bacteria through gels in the presence of IUCD monofilament tails. Contraception, 39, 205-216, 1989. (http://dx.doi.org/10.1016/S0010-7824(89)80009-5). 13. Batts, A.H., Marriott, C., Martin, G.P. and Bond, S.W. The effect of some preservatives used in nasal preparations on mucociliary clearance. J. Pharm. Pharmacol., 41, 156-159, 1989. (http://dx.doi.org/ 10.1111/j.2042-7158.1989.tb06420.x). 14. Wilkins, K.M., Martin, G.P., Hanlon, G.W. and Marriott, C. The influence of critical surface tension and microrugosity on the adhesion of bacteria to polymer monofilaments. Int. J. Pharm., 57, 1-7, 1989. (http://dx.doi.org/10.1016/0378-5173(89)90256-1). 15. Martin, G.P. and Marriott, C. The influence of a new hydrocolloid dermatological patch upon the blanching response induced by topical corticosteroid formulations. Curr. Ther. Res., 46, 828-836, 1989. 16. Wilkins, K.M., Hanlon, G.W., Martin, G.P. and Marriott, C. The role of adhesion in the migration of bacteria along intrauterine contraceptive device polymer monofilaments. Int. J. Pharm., 58, 164-174, 1990. (http://dx.doi.org/10.1016/0378-5173(90)90254-2). 17. Martin, G.P., Loveday, B.E. and Marriott, C. The effect of bromhexine hydrochloride on the viscoelastic properties of mucus from the mini-pig. Eur. Resp. J., 3, 392-396, 1990. 18. Batts, A.H., Marriott, C., Martin, G.P., Woods, C.F. and Bond, S.W. The effect of some preservatives used in nasal preparations on the mucus and ciliary components of mucociliary clearance. J. Pharm. Pharmacol., 42, 145-151, 1990. (http://dx.doi.org/ 10.1111/j.2042-7158.1990.tb05374.x). 19. Gizurarson, S., Marriott, C., Martin G.P. and Bechgaard, E. The influence of insulin and some excipients used in nasal insulin preparations on mucociliary clearance. Int. J. Pharm., 65, 243-247, 1990. (http://dx.doi.org/10.1016/0378-5173(90)90149-X). 20. Batts, A.H., Marriott, C., Martin, G.P., Bond, S.W., Greaves, J.L. and Wilson, C.G. The use of radiolabelled saccharin solution to monitor the effect of the preservatives Thiomersal, Benzalkonium chloride and EDTA on human nasal clearance. J. Pharm. Pharmacol., 43, 180-185, 1991. http://dx.doi.org/10.1016/0378-5173(90)90149-X 10.1111/j.2042-7158.1991.tb06662.x). 21. Hollingsbee, D.A., Fairbrother, J.E., Martin, G.P., Marriott, C. and Monger, L. The effect of a hydrocolloid dermatological patch (Actiderm) in potentiating the skin blanching activity of triamcinolone acetonide. Int. J. Pharm., 77, 199-209, 1991. (http://dx.doi.org/10.1016/0378-5173(91)90318-I). 22. Martin, G.P., El-Hariri, L.M., and Marriott, C. Bile salt- and lysophosphatidylcholine-induced membrane damage in human erythrocytes. J. Pharm. Phamacol., 44, 646-650, 1992. (http://dx.doi.org/10.1111/j.2042-7158.1992.tb05486.x). 23. El-Hariri, L.M., Marriott, C., and Martin, G.P. The mitigating effects of phosphatidylcholines on bile salt- and lysophosphatidylcholineinduced membrane damage. J. Pharm. Pharmacol., 44, 651-654, 1992. (http://dx.doi.org/10.1111/j.2042-7158.1992.tb05487.x). 24. Martin, G.P., Loveday, B.E. and Marriott, C. Bromhexine plus oxytetracycline: The effect of combined administration upon the rheological properties of mucus from the mini-pig. J. Pharm. Pharmacol., 45, 126-130, 1993. (http://dx.doi.org/10.1111/j.2042-7158.1993.tb03696.x). 25. Waring, M.J., Monger, L., Hollingsbee, D.A., Martin, G.P. and Marriott, C. Assessment of corticosteroid-induced skin blanching: evaluation of the Minolta Chromameter CR200. Int. J. Pharm., 94, 211-222, 1993. (http://dx.doi.org/10.1016/0378-5173(93)90026-C). 26. Bilbruck, J., Hanlon, G.W. and Martin, G.P. The effects of polyHEMA coating on the adhesion of bacteria to polymer monofilaments. Int. J. Pharm., 99, 293-301, 1993. (http://dx.doi.org/10.1016/0378-5173(93)90372-M). 27. Graham, A., Hasani, A., Alton, E.W.F.W., Martin, G.P., Marriott, C., Hodson, M.E., Geddes, D.M. No added benefit from nebulized amiloride in patients with cystic fibrosis. Eur. Resp. J., 6, 1243-1248, 1993. 28. Timsina, M.P., Martin, G.P., Marriott, C., Ganderton, D. and Yianneskis, M. Drug delivery to the respiratory tract using dry powder inhalers. Int. J. Pharm., 101, 1-13, 1994. (http://dx.doi.org/10.1016/0378-5173(94)90070-1). 29. Martin, G.P., Onyechi, J.O. and Marriott, C. Future prospects for the pulmonary delivery of drugs. S.T.P. Pharma Sciences, 4, 5-10, 1994. 30. Bilbruck, J., Hanlon, G.W., Gard, P.R. and Martin, G.P. The effects of transcervical monofilament insertion on the microbial status of the uterus in guinea pigs. J. Pharm. Pharmacol., 46, 213-216, 1994. (http://dx.doi.org/10.1111/j.2042-7158.1994.tb03781.x). 31. Lloyd, A., Martin, G.P. and Soozandehfar, S.H. Azopolymers: A means of colon specific drug delivery? Int. J. Pharm., 106, 255-260, 1994. (http://dx.doi.org/10.1016/0378-5173(94)90011-6). 32. Zeng, X.M., Martin, G.P. and Marriott, C. Albumin microspheres as a means of drug delivery to the lung: analysis of the effects of process variables on particle size using factorial design methodology. Int. J. Pharm., 107, 205-210, 1994. (http://dx.doi.org/10.1016/0378-5173(94)90435-9). 33. Zeng, X.M., Martin, G.P. and Marriott, C. Tetrandrine delivery to the lung: the optimisation of albumin microsphere preparation by central composite design. Int. J. Pharm., 109, 135-145, 1994. (http://dx.doi.org/10.1016/0378-5173(94)90141-4). 34. Onyechi, J.O., Martin, G.P., Marriott, C. and Murphy, L. Deposition of dry powder aerosols in cascade impactors at different flow rates. J. Aerosol Med., 7, 181-184, 1994. (http://dx.doi.org/10.1089/jam.1994.7.181). 35. Haghpanah, M., Marriott, C. and Martin, G.P. Potential use of microencapsulation for sustained drug delivery to the respiratory tract. J. Aerosol Med., 7, 185-188, 1994. (http://dx.doi.org/10.1089/jam.1994.7.185). 36. Shafi, Z.B., Martin, G.P., Olliff, C.J. and James, S.L. Surface modification of albumin microspheres. J. Drug Targeting, 3, 53-56, 1995. (http://dx.doi.org/10.3109/10611869509015933). 37. Zeng, X.M., Martin, G.P. and Marriott, C. Preparation and in vitro evaluation of tetrandrine-entrapped albumin microspheres as an inhaled drug delivery system. Eur. J. Pharm. Sci., 3, 87-93, 1995. (http://dx.doi.org/10.1016/0928-0987(94)00078-E). 38. Zeng, X.M., Martin, G.P. and Marriott, C. The controlled delivery of drugs to the lung. Int. J. Pharm., 124, 149-164, 1995. (http://dx.doi.org/10.1016/0378-5173(95)00104-Q). 39. Brown, M.B., Marriott, C. and Martin, G.P. The effect of hyaluronan on the in vitro deposition of diclofenac within the skin. Int. J. Tissue React., 17, 133-140, 1995. 40. Pritchard, K., Lansley, A.B., Martin, G.P., Helliwell, M., Marriott, C. and Benedetti, L.M. Evaluation of the bioadhesive properties of hyaluronan derivatives: Detachment weight and mucociliary transport rate studies. Int. J. Pharm., 129, 137-145, 1996. (http://dx.doi.org/10.1016/0378-5173(95)04280-6). 41. Ladenheim, D., Martin, G.P., Marriott, C., Hollingsbee, D.A. and Brown, M.B. An in vitro study of the effect of hydrocolloid patch occlusion on the penetration of triamcinolone acetonide through human skin. J. Pharm. Pharmacol., 48, 806-811, 1996. (http://dx.doi.org/10.1111/j.2042-7158.1996.tb03978.x). 42. MacAdam, A.B., Shafi, Z.B., James, S.L., Marriott, C. and Martin, G.P. Preparation of hydrophobic and hydrophilic albumin microspheres and determination of surface carboxylic acid and amino residues. Int. J. Pharm., 151, 47-55, 1997. (http://dx.doi.org/10.1016/S0378-5173(97)04886-2). 43. Needleman, I.G., Martin, G.P. and Smales, F.C. An investigation of bioadhesion for periodontal and oral mucosal drug delivery. J. Clin. Periodontol., 24, 394-400, 1997. (http://dx.doi.org/10.1111/j.1600-051X.1997.tb00203.x). 44. Bragger, J.L., Lloyd, A.W., Soozandehfar, S.H., Bloomfield, S.F., Marriott, C. and Martin, G.P. Investigations into the azo reducing activity of a common colonic microorganism. Int. J. Pharm., 157, 61-71, 1997. (http://dx.doi.org/10.1016/S0378-5173(97)00214-7). 45. Nazir, T., Gould, L.A., Marriott, C., Martin, G.P. and Brown, M.B. High performance liquid chromatography of a cyclosporin A formulation on a porous graphitic carbon column. Chromatographia, 46, 628-636, 1997. (http://dx.doi.org/10.1007/BF02490523). 46. Needleman, I.G., Martin, G.P. and Smales, F.C. Characterisation of bioadhesives for periodontal and oral mucosal delivery. J. Periodontol Res., 25, 74-82, 1998. (http://dx.doi.org/10.1111/j.1600-051X.1998.tb02366.x). 47. Srichana, T., Martin, G.P. and Marriott, C. On the relationship between drug and carrier deposition from dry powder inhalers in vitro. Int. J. Pharm., 167, 13-23, 1998. (http://dx.doi.org/10.1016/S0378-5173(98)00037-4). 48. Srichana, T., Martin, G.P. and Marriott, C. Dry powder inhalers: the influence of device resistance and powder formulation on drug and lactose deposition in vitro. Eur. J. Pharm. Sci., 7, 73-80, 1998. (http://dx.doi.org/10.1016/S0928-0987(98)00008-6). 49. Zeng, X.M., Martin, G.P., Tee, S. and Marriott, C. The role of fine particle lactose on the dispersion and deaggregation of salbutamol sulphate in an air stream in vitro. Int. J. Pharm., 176, 99-110, 1998. (http://dx.doi.org/10.1016/S0378-5173(98)00300-7). 50. Martin, G.P., Brown, M.B., Bennett, F.C. and Marriott, C. An in vitro study of the diclofenac delivery properties of hyaluronan in human skin. Drug Delivery, 6, 39-43, 1999. (http://dx.doi.org/10.1080/107175499267147). 51. Zeng, X.M., Martin, G.P., Tee, S., Ghoush, A.A. and Marriott, C. Effects of particle size and adding sequence of fine lactose on the deposition of salbutamol sulphate from a dry powder formulation. Int. J. Pharm., 182, 133-144, 1999. (http://dx.doi.org/10.1016/S0378-5173(99)00021-6). 52. Larhrib, H., Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. The use of different grades of lactose as a carrier for aerosolised salbutamol sulphate. Int. J. Pharm., 191, 1-14, 1999. (http://dx.doi.org/10.1016/S0378-5173(99)00164-7). 53. Martin, G.P., Ladenheim, D., Marriott, C., Hollingsbee, D.A. and Brown, M.B. The influence of hydrocolloid patch composition on the bioavailability of triamcinolone acetonide in humans. Drug Develop. Indust. Pharm., 26, 35-43, 2000. (http://dx.doi.org/10.1081/DDC-100100325). 54. MacAdam, A.B., Shafi, Z.B., Marriott, C., Martin, G.P. and James, S.L. Anti-mucus polyclonal antibody production, purification and linkage to the surface of albumin microspheres. Int. J. Pharm., 195, 147-158, 2000. (http://dx.doi.org/10.1016/S0378-5173(99)00390-7). 55. Srichana, T., Brain, A., Marriott, C. and Martin, G.P. A study of drug-carrier-interactions in dry powder inhaler formulations using the Andersen cascade impactor, X-ray microanalysis and time of flight aerosol beam spectrometry. Chem. Pharm. Bull., 48, 167-174, 2000. 56. Suedee, R., Srichana, T. and Martin, G.P. Evaluation of matrices containing molecularly imprinted polymers in the entioselectivecontrolled delivery of -blockers. J. Contr. Rel., 66, 135-147, 2000. (http://dx.doi.org/10.1016/S0168-3659(99)00261-8). 57. Zeng, X.M., Pandhal, K.H. and Martin, G.P. The influence of lactose carrier on the content homogeneity and dispersibility of beclomethasone diproprionate from dry powder aerosols. Int. J. Pharm., 197, 41-52, 2000. (http://dx.doi.org/10.1016/S0378-5173(99)00400-7). 58. Soozandehfar, S.H., Bragger, J.L., Martin, G.P. and Lloyd, A.W. Synthesis and bacterial degradation of azopolymers. Int. J. Pharm., 198, 71-82, 2000. (http://dx.doi.org/10.1016/S0378-5173(99)00454-8). 59. Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. The influence of the carrier morphology on the drug delivery by dry powder inhalers. Int J. Pharm., 200, 93-106, 2000. (http://dx.doi.org/10.1016/S0378-5173(00)00347-1). 60. Lim, S.T., Martin, G.P., Berry, D.J. and Brown, M.B. Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel microspheres of hyaluronic acid and chitosan. J. Contr. Rel.,66, 281-292, 2000. (http://dx.doi.org/10.1016/S0168-3659(99)00285-0). 61. Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. Crystallization of lactose from Carbopol gels. Pharm. Res., 17, 879-886, 2000. (http://dx.doi.org/10.1023/A:1007572612308). 62. Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. The influence of crystallization conditions on the morphology of lactose intended for use as a carrier for dry powder aerosols. J. Pharm. Pharmacol., 52, 633-643, 2000. (http://dx.doi.org/10.1211/0022357001774462). 63. McDonald K.J. and Martin, G.P. Transition to CFC-free metered dose inhalers - into the new millennium. Int. J. Pharm., 201, 89-107, 2000. (http://dx.doi.org/10.1016/S0378-5173(00)00401-4). 64. Srichana, T., Martin, G.P. and Marriott, C. A human oral-throat cast integrated with a twin-stage impinger for evaluation of dry powder inhalers. J. Pharm. Pharmacol., 52, 771-778, 2000. (http://dx.doi.org/10.1211/0022357001774624). 65. Gould, L.A., Lansley, A.B., Brown, M.B., Forbes, B.J. and Martin, G.P. Mitigation of surfactant erythrocyte toxicity by egg phosphatidylcholine. J. Pharm. Pharmacol., 52, 1203-1209, 2000. (http://dx.doi.org/10.1211/0022357001777333). 66. Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. The effects of carrier size and morphology on the dispersion of salbutamol sulphate after aerosolisation at different flow rates. J. Pharm. Pharmacol., 52, 1211-1221, 2000. (http://dx.doi.org/10.1211/0022357001777342). 67. Tee, S., Zeng, X.M., Marriott, C. and Martin, G.P. The use of different sugars as fine and coarse carriers for aerosolised salbutamol delivery. Int. J. Pharm., 208, 111-123, 2000. (http://dx.doi.org/10.1016/S0378-5173(00)00553-6). 68. Needleman, I., Mather, S.J., Martin, G.P. and Sobnack, R. Periodontal pocket clearance by gamma scintigraphy in human volunteers. J. Clin. Periodontol., 27, 904-909, 2000. (http://dx.doi.org/10.1034/j.1600-051x.2000.027012904.x). 69. Forbes, B., Hashmi, N., Martin, G.P. and Lansley, A.L. Formulation of inhaled medicines: effect of delivery vehicle on immortalised epithelial cells. J. Aerosol Med., 13, 281-288, 2000. (http://dx.doi.org/10.1089/jam.2000.13.281). 70. Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. The use of lactose recrystallised from Carbopol gels as a carrier for aerosolised salbutamol sulphate. Eur. J. Pharm. Biopharm., 51, 55-62, 2001. (http://dx.doi.org/10.1016/S0939-6411(00)00142-9). 71. Liao, Y.H., Brown, M.B. and Martin, G.P. Turbidimetric and HPLC assays for the determination of formulated lysozyme activity. J. Pharm. Pharmacol., 53, 549-554, 2001. (http://dx.doi.org/10.1211/0022357011775668). 72. Amr, S.K., Brown, M.B., Martin, G.P. and Forbes, B. Activation of clindamycin phosphate by human skin. J. Applied Microbiol., 90, 550-554, 2001. (http://dx.doi.org/10.1046/j.1365-2672.2001.01282.x). 73. Zeng, X.M., Martin, G.P. and Marriott, C. Effects of molecular weight of polyvinylpyrrolidone on the glass transition and crystallization of co-lyophilized sucrose. Int. J. Pharm., 218, 63-73, 2001. (http://dx.doi.org/10.1016/S0378-5173(01)00613-5). 74. Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. Lactose as a carrier in dry powder formulations: the influence of particle characteristics on drug delivery. J. Pharm. Sci., 90, 1424-1434, 2001. (http://dx.doi.org/10.1002/jps.1094). 75. Brown, M.B., Hanpanitcharoen, M. and Martin, G.P. An in vitro investigation into the effect of glycosaminoglycans on the skin partitioning and deposition of NSAIDs. Int. J. Pharm., 225, 113-121, 2001. (http://dx.doi.org/10.1016/S0378-5173(01)00758-X). 76. Lim, S.T., Forbes, B., Martin, G.P. and Brown M.B. In vivo and in vitro characterization of novel microparticulates based on hyaluronan and chitosan hydroglutamate. AAPS PharmSciTech., 2(4), article 20, 2001. 77. Lim, S.T., Martin, G.P., Forbes, B., Berry, D.J. and Brown, M.B. In vivo evaluation of novel hyaluronan/chitosan microparticulate delivery systems for the nasal delivery of gentamycin in rabbits. Int. J. Pharm., 231, 73-82, 2001. (http://dx.doi.org/10.1016/S0378-5173(01)00873-0). 78. Forbes, B., Lim, S., Martin, G.P. and Brown, M.B. An in vitro technique for evaluating inhaled nasal delivery systems. S.T.P. Pharma Sciences, 12, 75-79, 2002. 79. Suedee, R., Srichana, T., Chotivatesin, R. and Martin, G.P. Stereoselective-release behaviors and microscopic of imprinted bead matrices. Drug Dev. Ind. Pharm., 28, 545-554, 2002. (http://dx.doi.org/10.1081/DDC-120003450). 80. Liao, Y.H., Brown, M.B., Nazir, T., Quader, A. and Martin, G.P. Effects of sucrose and trehalose on the preservation of the native structure of spray-dried lysozyme. Pharm. Res., 19, 1847-1853, 2002. (http://dx.doi.org/10.1023/A:1021445608807). 81. Liao, Y.H., Brown, M.B., Quader, A. and Martin, G.P. Protective mechanism of stabilising excipients against dehydration in the freeze-drying of proteins. Pharm. Res., 19, 1854-1861, 2002. (http://dx.doi.org/10.1023/A:1021497625645). 82. Forbes, B., Shah, A., Martin, G.P. and Lansley, A.B. The human bronchial epithelial cell line 16HBE14o- as a model system of the airways for studying drug transport. Int. J. Pharm., 257, 161-167, 2003. (http://dx.doi.org/10.1016/S0378-5173(03)00129-7). 83. Larhrib, H., Martin, G.P., Marriott, C. and Prime, D. The influence of carrier and drug morphology on drug delivery from dry powder formulations. Int. J. Pharm., 257, 283-296, 2003. (http://dx.doi.org/10.1016/S0378-5173(03)00156-X). 84. Larhrib, H., Prime, D., Martin, G.P. and Marriott, C. Characterisation and deposition studies of engineered lactose crystals with potential for use as carrier for aerosolized salbutamol sulphate. Eur. J. Pharm. Sci., 19, 211-221, 2003. (http://dx.doi.org/10.1016/S0928-0987(03)00105-2). 85. Liao, Y-H., Brown, M.B., Quader, A. and Martin, G.P. Investigation of the physical properties of spray-dried stabilized lysozyme particles. J. Pharm. Pharmacol., 55, 1213-1221, 2003. (http://dx.doi.org/10.1211/0022357021611). 86. Yff, B., Royall, P.G., Brown, M.B. and Martin, G.P. An investigation of calibration methods for solution calorimetry. Int. J. Pharm., 269, 361-372, 2004. (http://dx.doi.org/10.1016/j.ijpharm.2003.09.01). 87. Akomeah, F., Nazir, T., Martin, G.P. and Brown, M.B. Effect of heat on the percutaneous absorption and skin retention of three model penetrants. Eur. J. Pharm. Sci., 21, 337-345, 2004. (http://dx.doi.org/10.1016/j.ejps.2003.10.02). 88. Jones, S.A., Brown, M.B. and Martin, G.P. Determination of polyvinyl alcohol using gel filtration liquid chromatography. Chromatographia, 59, 43-46, 2004. (http://dx.doi.org/10.1016/j.jpba.2004.01.02). 89. Jones, S.A., Brown, M.B. and Martin, G.P. Determination of polyvinylpyrrolidone using high performance liquid chromatography. J. Pharm. Biomed. Anal., 35, 621-624, 2004. (http://dx.doi.org/10.1016/j.jpba.2004.01.02). 90. Liao, Y-H., Brown, M.B. and Martin, G.P. Investigation of the stabilisation of freeze-dried lysozyme and the physical properties of the formulations. Eur. J. Pharm. Biopharm., 58, 15-24, 2004. (http://dx.doi.org/10.1016/j.ejpb.2004.03.02). 91. Zaman, M.A., Martin, G.P., Rees, G.D. and Royall, P.G. The effect of hydration on the thermal behaviour of hydrophilic non-aqueous gels stabilized by Carbopol 974P. Thermochim Acta, 417, 251-255, 2004. (http://dx.doi.org/10.1016/j.tca.2003.09.03). 92. Jones, S.A., Martin, G.P., Brown, M.B. High-pressure aerosol suspensions – a novel laser diffraction particle sizing system for hydroalkane metered dose inhalers. Int. J. Pharm., 302, 154-165, 2005. (http://dx.doi.org/10.1016/j.ijpharm.2005.07.00). 93. Jones, S.A., Martin, G.P., Royall, P.G., Brown, M.B. Biocompatible polymer blends: the effects of physical processing on the molecular interaction of poly(vinyl alcohol) and poly(vinylpyrrolidone). Journal of Applied Polymer Science, 98, 2290-2299, 2005. (http://dx.doi.org/ 10.1002/app.22390). 94. Liao, Y-H., Brown, M.B., Jones, S.A., Nazir, T. and Martin, G.P. The effects of polyvinyl alcohol on the physical stability of spray-dried proteins particles within surfactant-free HFA 134a-based pressurised metered dose inhalers. Int. J. Pharm., 304, 29-39, 2005. (http://dx.doi.org/10.1016/j.ijpharm.2005.07.013). 95. Liao, Y-H., Jones, S.A., Forbes, B., Martin, G.P. and Brown, M.B. Hyaluronan: pharmaceutical characterisation and drug delivery. Drug Delivery, 12, 327-342, 2005. (http://dx.doi.org/10.1080/10717540590952555). 96. Jelvehgari, M., Siahi-Shadbad, M.R., Azarmi, S., Martin, G.P. and Nokhodchi, A. The microsponge delivery system of benzoyl peroxide: characterization and release studies. Int. J. Pharm., 308, 124-132, 2006. (http://dx.doi.org/10.1016/j.ijpharm.2005.11.001). 97. Murnane, D., Martin, G.P. and Marriott, C. Validation of a reverse-phase high performance liquid chromatographic method for concurrent assay of a weak base (salmeterol xinafoate) and a pharmacologically active steroid (fluticasone propionate). J. Pharm. Biomed. Anal., 40, 1149-1154, 2006. (http://dx.doi.org/10.1016/j.jpba.2005.09.028). 98. Brown, M.B., Martin, G.P., Jones, S.A. and Akomeah, F.K. Dermal and transdermal drug delivery systems; current and future prospects. Drug Delivery, 13, 175-187, 2006. (http://dx.doi.org/10.1080/10717540500455975). 99. Bodhibukkana, C., Srichana, T., Kaewnopparat, S., Tangthong, N., Bouking, P., Martin, G.P. and Suedee, R. Composite membrane of bacterially-derived cellulose and molecularly imprinted polymer for use as a transdermal enantioselective controlled-release system of racemic propranolol. J. Control. Rel., 113, 43-56, 2006. (http://dx.doi.org/10.1016/j.jconrel.2006.03.007). 100. Suedee, R., Seechamnanturakit, V., Canyuk, B., Ovatlarnporn C. and Martin, G.P. Temperature sensitive dopamine-imprinted (N,N-methylene-bis-acrylamide cross-linked) polymer and its potential application to the selective extraction of adrenergic drugs from urine. J. Chromatography A, 1114, 239-249, 2006. (http://dx.doi.org/10.1016/j.chroma.2006.02.033). 101. Jones, S.A., Martin, G.P. and Brown, M.B. Manipulation of beclomethasone-hydrofluoroalkane interactions using biocompatible macromolecules. J. Pharm. Sci, 95, 1060-1074, 2006. (http://dx.doi.org/10.1002/jps.20608). 102. Grainger, C.I., Greenwell, L.L., Lockley, D.J., Martin, G.P. and Forbes, B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm. Res., 23, 1482-1490, 2006. (http://dx.doi.org/10.1007/s11095-006-0255-0). 103. Jones, S.A., Martin, G.P. and Brown, M.B. Stabilisation of deoxyribonuclease in hydrofluoroalkanes using miscible vinyl polymers. J. Control. Rel., 115, 1-8, 2006. (http://dx.doi.org/10.1016/j.jconrel.2006.06.003). 104. Zeng, X.M., MacRitchie, H.B., Marriott, C. and Martin, G.P. Correlation between inertial impaction and laser diffraction sizing data for aerosolized carrier-based dry powder formulations. Pharm. Res., 23, 2200-2209, 2006. (http://dx.doi.org/10.1007/s11095-006-9055-9). 105. Martin, G.P., MacRitchie, H.B., Marriott, C. and Zeng, X.M. Characterisation of a carrier-free dry powder aerosol formulation using inertial impaction and laser diffraction. Pharm. Res., 23, 2210-2219, 2006. (http://dx.doi.org/10.1007/s11095-006-9056-8). 106. Marriott, C., MacRitchie, H.B., Zeng, X.M. and Martin, G.P. Development of a laser diffraction method for the determination of the particle size of aerosolised powder formulations. Int. J.Pharm., 326, 39-49, 2006. (http://dx.doi.org/10.1016/j.ijpharm.2006.07.021). 107. Martin, G.P., Marriott, C. and Zeng, X.M. Influence of realistic inspiratory flow profiles on fine particle fractions of dry powder aerosol formulations. Pharm. Res., 24, 361-369, 2007. (http://dx.doi.org/10.1007/s11095-006-9156-5). 108. Zeng, X.M., MacRitchie, H.B., Martin, G.P. and Marriott, C. Humidity-induced changes in the aerodynamic properties of dry powder aerosol formulations containing different carriers. Int. J. Pharm., 333, 45-55, 2007. (http://dx.doi.org/10.1016/j.ijpharm.2006.09.048). 109. Akomeah, F.K., Martin, G.P. and Brown, M.B. Variability in human skin permeability in vitro: comparing penetrants with different physicochemical properties. J. Pharm Sci., 96, 824-834, 2007. (http://dx.doi.org/10.1002/jps.20773). 110. Grenha, A., Grainger, C., Dailey, L.A., Seijo, B., Martin, G.P., Remuñán-López, C., Forbes, B. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur. J. Pharm. Sci., 31, 73-84, 2007. (http://dx.doi.org/10.1016/j.ejps.2007.02.008). 111. Maghsoodi, M., Hassan-Zadeh, D., Barzegar-Jalali, M., Nokhodchi, A., Martin, G.P. Improved compaction and packing properties of naproxen agglomerated crystals obtained by spherical crystallization technique. Drug Devel. Ind. Pharm., 33, 1216-1224, 2007. (http://dx.doi.org/10.1080/03639040701377730). 112. Jantarat, C., Tangthong, N., Songkro, S., Martin, G.P. and Suedee, R. S-propranolol imprinted polymer nanoparticle-on-microsphere composite porous cellulose membrane for the enantioselectively controlled delivery of racemic propranolol. Int. J. Pharm., 349, 212-225, 2008. (http://dx.doi.org/10.1016/j.ijpharm.2007.07.030). 113. Maghsoodi, M., Taghizadeh, O., Martin, G.P. and Nokhodchi, A. Particle design of naproxen-disintegrant agglomerates for direct compression by a crystallo-co-agglomeration technique. Int. J. Pharm., 351, 45-54, 2008. (http://dx.doi.org/10.1016/j.ijpharm.2007.09.033). 114. Akomeah, F.K., Martin, G.P., Muddle, A.G. and Brown, M.B. Effect of abrasion induced by a rotating brush on the skin permeation of solutes with varying physicochemical properties. Eur. J. Pharm. Biopharm., 68, 724-734, 2008. (http://dx.doi.org/10.1016/j.ejpb.2007.06.005). 115. Murnane, D., Marriott, C. and Martin, G.P. Developing an environmentally benign process for the production of microparticles: amphiphilic crystallization. Eur. J. Pharm. Biopharm., 69, 72-82, 2008. (http://dx.doi.org/10.1016/j.ejpb.2007.10.014). 116. Murnane, D., Marriott, C. and Martin, G.P. Comparison of salmeterol xinafoate microparticle production by conventional and novel antisolvent crystallization. Eur. J. Pharm. Biopharm., 69, 94-105, 2008. (http://dx.doi.org/10.1016/j.ejpb.2007.09.016). 117. Zaman, M.A., Martin, G.P. and Rees, G.D. Mucoadhesion, hydration and rheological properties of non-aqueous delivery systems (NADS) for the oral cavity. J. Dent. 36, 351-359, 2008. (http://dx.doi.org/10.1016/j.jdent.2008.01.014). 118. Murnane, D., Marriott, C. and Martin, G.P. In situ and ex situ analysis of salmeterol xinafoate microcrystal formation from poly(ethylene glycol) 400-water cosolvent mixtures. Cryst. Growth Des. 8, 1855-1862, 2008. (http://dx.doi.org/10.1021/cg700953k). 119. Murnane, D., Marriott, C. and Martin, G.P. Polymorphic control of inhalation microparticles prepared by crystallization. Int. J. Pharm., 361,141–149, 2008. (http://dx.doi.org/10.1016/j.ijpharm.2008.05.033). 120. Murnane, D., Marriott, C. and Martin, G.P. Crystallization and crystallinity of fluticasone propionate. Cryst. Growth Des. 8, 2753-2764, 2008. (http://dx.doi.org /10.1021/cg700954t). 121. Traynor, M.J., Brown, M.B., Pannala, A., Beck, P. and Martin, G.P. Influence of alcohol on the release of tramadol from controlled release formulations during in vitro dissolution experiments. Drug Devel. Ind. Pharm., 34:885–889, 2008. (http://dx.doi.org/10.1080/03639040801929240). 122. Murnane, D., Martin, G.P. and Marriott, C. Investigations into the formulation of metered dose inhalers of salmeterol xinafoate and fluticasone propionate microcrystals. Pharm. Res., 25, 2283-2291, 2008. (http://dx.doi.org/10.1007/s11095-008-9622-3). 123. Murnane, D., Martin, G.P. and Marriott, C. Dry powder formulations for inhalation of fluticasone propionate and salmeterol xinafoate microcrystals. J. Pharm. Sci., 98, 503-515, 2009. (http://dx.doi.org/10.1002/jps.21450). 124. Grainger, C.I., Greenwell, L.L., Martin, G.P., Forbes B. The permeability of large molecular weight solutes following particle delivery to airinterfaced cells that model the respiratory mucosa Eur. J. Pharm. Biopharm., 71, 318-324, 2009. (http://dx.doi.org/10.1016/j.ejpb.2008.09.006). 125. Akomeah, F.K., Martin, G.P. and Brown, M.B. Short-term (post)iontophoretic transport of model penetrants across excised human epidermis. Int. J. Pharm., 367, 162-168, 2009. (http://dx.doi.org/10.1016/j.ijpharm.2008.09.041). 126. Heikal, L., Martin, G.P. and Dailey, L.A. Characterization of the decomposition behaviour of S-nitrosoglutathione and a new class of analogues: S-nitrosophytochelatins Nitric Oxide, 20, 157-165, 2009. (http://dx.doi.org/10.1016/j.niox.2008.11.001) 127. Pangsomboon, K., Bansal, S., Martin, G.P., Suntinanalert, P., Kaenewopopparat, S. and Srichana, T. Bacteriocin produced by Lactobacillus paracasei HL32: its mode of action, haemolytic, biological activity and stability. J. Appl. Microbiol., 106, 1928-1940, 2009. (http://dx.doi.org/10.1111/j.1365-2672.2009.04146.x). 128. Belhadj Salem, L., Bosquillon, C., Dailey, L.A., Delattre, L., Martin, G.P., Evrard, B. and Forbes, B. Sparing methylation of β-cyclodextrin mitigates cytotoxicity and permeability induction in respiratory epithelial cell layers in vitro. J. Control. Rel., 136, 110-116, 2009. (http://dx.doi.org/10.1016/j.jconrel.2009.01.019). 129. Taki, M., Marriott, C., Zeng, X.M. and Martin, G.P. Aerodynamic Deposition of Combination Dry Powder Inhaler Formulations in vitro: a comparison of three impactors. Int. J. Pharm., 388, 40–51, 2010. (http://dx.doi.org/10.1016/j.ijpharm.2009.12.031). 130. Kaialy, W., Martin, G.P., Ticehurst, M., Momin, M.N. and Nokhodchi, A. The enhanced aerosol performance of salbutamol from dry powders containing engineered mannitol as excipient. Int. J. Pharm., 392, 178-188, 2010. (http://dx.doi.org/10.1016/j.ijpharm.2010.03.057). 131. Sakeer, K., Al-Zein, H., Martin, G.P. and Nokhodchi, A. Use of xanthan and its binary blends with synthetic polymers to design controlled release formulations of buccoadhesive nystatin tablets. Pharm. Dev. Technol., 15, 360-368, 2010. (http://dx.doi.org/10.1080/10837450903246416). 132. Zaman, M.A., Martin, G.P., Rees, G.D. Bioadhesion and retention of non-aqueous drug delivery systems (NADDS) for administration to dental hard tissues. J. Dent., 38, 757-767, 2010. (http://dx.doi.org/10.1016/j.jdent.2010.06.005). 133. Kaialy, W., Martin, G.P., Ticehurst, M.D., Royall, P., Mohammed, M.A. and Nokhodchi, A. Characterisation and deposition studies of recrystallised lactose from binary mixtures of ethanol/butanol for improved drug delivery from dry powder inhalers. AAPS J., 13, 30-43, 2011. (http://dx.doi.org/10.1208/s12248-010-9241-x). 134. Taki, M., Marriott, C. Zeng, X.M. and Martin, G.P. The production of ‘aerodynamically equivalent’ drug and excipient inhalable powders using a novel fractionation technique. Eur. J. Pharm. Biopharm., 77, 283-296, 2011. (http://dx.doi.org/10.1016/j.ejpb.2010.11.021). 135. Taki, M., Ahmed, S., Marriott, C., Zeng, X.M. and Martin, G.P. The 'stage-by-stage' deposition of drugs from commercial single-active and combination dry powder inhaler formulations. Eur. J. Pharm. Sci., 43, 225-235, 2011. (http://dx.doi.org/10.1016/j.ejps.2011.04.014). 136. Heikal, L., Aaronson, P., Ferro, A., Nandi, M., Martin, G.P. and Dailey, L.A S-nitrosophytochelatins: investigation of the bioactivity of an oligopeptide nitric oxide delivery system. Biomacromolecules, 12, 2103-2113, 2011. (http://dx.doi.org/10.1021/bm200159h). 137. Moss, G.P., Y., Wilkinson, S.C., Davey, N., Adams, R., Martin, G.P., Prapopolou, M. and Brown, M.B. The application and limitations of mathematical modeling in the prediction of permeability across mammalian skin and polydimethylsiloxane membranes. J. Pharm. Pharmac., 63, 1411-1427, 2011. (http:dx.doi.org/10.1111/j.2042-7158.2011.01345.x). 138. Royall, P.G., Woodhead, B., Tang, S.J., Martin, G.P., Stockton, B.M. and Murnane, D. Formation and measurement of process induced disorder during the manufacture of inhalation medicines. J. Drug Del., Sci. Tech., 21, 311-318, 2011. 139. Taki, M., Esmaeili, F. and Martin, G.P. The scientific basis and challenges of combination inhaled products. J. Drug Del., Sci. Tech., 21, 293-300, 2011. 140. Sadler, R.C., Prime, D., Burnell, P.K., Martin, G.P. and Forbes, B. Integrated in vitro experimental modelling of inhaled drug delivery: deposition, dissolution and absorption. J. Drug Del., Sci. Tech., 21, 331-338, 2011. 141. Kaialy, W., Martin, G.P., Larhrib, H., Ticehurst, M.D., Kolosioneka, E., Nokhodchi, A. The influence of physical properties and morphology of crystallised lactose on delivery of salbutamol sulphate from dry powder inhalers. Colloid Surface B, 89, 29-39, 2012. (http://dx.doi.org/10.1016/j.colsurfb.2011.08.019). 142. Zughaid, H., Forbes, B., Martin, G.P. and Patel, N. Bile salt composition is secondary to bile salt concentration in determining hydrocortisone and progesterone solubility in intestinal mimetic fluids. Int. J. Pharm., 422, 295–301, 2012. (http://dx.doi.org/10.1016/j.ijpharm.2011.11.012). 143. Jawad, R., Elleman, C., Vermeer, L., Drake, A.F., Woodhead, B., Martin, G.P. and Royall, P.G. The measurement of the β/α anomer composition within amorphous lactose prepared by spray and freeze drying using a simple 1H-NMR method. Pharm. Res., 29, 511-524, 2012. (http://dx.doi.org/10.1007/s11095-011-0575-6). 144. Grainger, C.I., Saunders, M., Buttini, F., Merolla, L.L., Martin, G.P., Jones, S.A. and Forbes, B. Critical characteristics for corticosteroid solution metered dose inhaler bioequivalence. Mol. Pharmaceutics, 9, 563-569, 2012. (http://dx.doi.org/10.1021/mp200415g). 145. Kaialy, W., Larhrib, H., Martin, G.P., Nokhodchi, A. The effect of engineered mannitol-lactose mixture on dry powder inhaler performance. Pharm. Res., 29, 2139-2156, 2012. (http://dx.doi.org/10.1007/s11095-012-0743-3). 146. Benaouda, F., Brown, M.B., Ganguly, S., Jones, S.A., Martin, G.P. Discriminating the molecular identity and function of discrete supramolecular structures in topical pharmaceutical formulations. Mol. Pharmaceutics, 9, 2505-2512, 2012. (http://dx.doi.org/10.1021/mp300127f). 147. Benaouda, F., Brown, M.B., Martin, G.P. and Jones, S.A. Triggered in situ drug supersaturation and hydrophilic matrix self-assembly. Pharm. Res., 29, 3434-3442, 2012. (http://dx.doi.org/10.1007/s11095-012-0838-x). 148. Benaouda, F., Brown, M.B., Shah, B., Martin, G.P. and Jones, S. A. The influence of self-assembling supramolecular structures on the passive membrane transport of ion-paired molecules. Int. J. Pharm., 439, 334-341, 2012. (http://dx.doi.org/10.1016/j.ijpharm.2012.09.001). Submitted for publication Gan, S.Y., Hider, R.C., Martin, G.P., Jones, S.A., Kong, X.L., Brown, M.B. Creeth, J.E., Makin, S.A. and Wade, W.G. Antibacterial activity of zinc enhanced by complexation with chelators. Wade, J.B., Martin, G.P. and Long, D.F. An assessment of powder pycnometry as a means of determining granule envelope density. Wade, J.B., Martin, G.P. and Long, D.F. The effect of moisture content on the compaction properties of hydroxyapatite (TRI-CAL WGTM). Heikal, L., Starr, A., Martin, G.P., Nandi, M. and Dailey, L.A. In vivo pharmacological activity and biodistribution of S-nitrosophytochelatins after intravenous and intranasal administration in mice. In Preparation Zughaid, H., Forbes, B.,Gary P. Martin, G.P. and Patel, N. Effect of intestinal mimetic fluids on drug solubility and permeability; additional effects of incorporation of lipid digestion products on drug solubility. (3) Contributions to edited works. 1. Batts, A.H., Marriott, C., Martin, G.P. and Bond, S.W. Mucociliary transport rate: the influence of preservatives. In Aiache, J.M. and Hirtz, J. (eds.) Proceedings of the third European congress of biopharmaceutics and pharmacokinetics, Freiburg, April 21-24, 1987, Vol. 1: 178-185. 2. Martin, G.P., Marriott, C. and Loveday, B.E. In vivo effect of bromhexine hydrochloride on the rheological properties of mucus. In Aiache, J.M. and Hirtz, J. (eds.) Proceedings of the third European congress of biopharmaceutics and pharmacokinetics, Freiburg, April 21-24, 1987, Vol. 1: 383-390. 3. Marriott, C. and Martin, G.P. Preclinical results of a new dermatological patch used in conjunction with topical corticosteroids in inducing the blanching response. In: Ryan T. J. ed: Beyond occlusion: wound care proceedings. International Congress and Symposium Series No. 136, London: Royal Society of Medicine Services Ltd., 1988: 25-34. Also in: Ryan, T.J. ed:Beyond Occlusion: dermatology proceedings. International Congress and Symposium Series, No. 137, London: Royal Society of Medicine Services Ltd., 1988: 9-18. 4. Walker, M., Hollingsbee, D., Hadgraft, J., Monger, L., Martin, G. and Marriott, C. Influence of oleic acid on the physical and chemical properties of the human epidermal barrier. In Scott, R.C., Guy, R.H., Hadgraft, J. and Boddé , H.E. (eds.) Prediction of Percutaneous Penetration - methods measurements modelling. Vol. 2. IBC Technical Services Ltd, London, 1991: 86-96. 5. Walker, M., Hollingsbee, D., Monger, L., Martin, G. and Marriott, C. In-vivo: in vitro comparisons of the percutaneous absorption of 0.1% halcinonide creams. In Scott, R.C., Guy, R.H., Hadgraft, J. and Boddé, H.E. (eds.) Prediction of Percutaneous Penetration - methods measurements modelling. Vol. 2. IBC Technical Services Ltd, London, 1991: 533-540. 6. Ladenheim, D, Martin, G.P., Marriott, C., Hollingsbee, D.A. and Fairbrother, J.E. In vitro and in vivo water vapour uptake characteristics of hydrocolloid-containing dermatological patches. In Scott, R.C., Guy, R.H., Hadgraft, J. and Boddé, H.E. (eds.) Prediction of Percutaneous Penetration - methods measurements modelling. Vol. 2. IBC Technical Services Ltd, London, 1991: 614-620. 7. Helliwell, M., Martin, G.P. and Marriott, C. Modification of surface carboxyl groups of microcapsules using a water-soluble carbodiimide. In: Microencapsulation of Drugs, Whateley, T.L. (ed.) (Drug Targeting and Delivery Series: Vol 1, Florence, A.T. and Gregoriadis, G. (Series eds.)), Harwood Academic Publishers, London, 1992: 65-80. 8. Martin, G.P. and Lloyd, A.W. Basic principles of liposomes for drug use. In Liposome Dermatics, Braun-Falco, O., Korting, H.C. and Maibach, H.I. (eds), Springer-Verlag, Berlin Heidelberg, 1992: 20-26. 9. Martin, G.P. Phospholipids as skin penetration enhancers. In Walters, K.A. and Hadgraft, J. (eds.), Pharmaceutical Skin Penetration Enhancement, (Drugs and the Pharmaceutical Sciences, Vol. 59, Swarbrick, J. (Series ed.)), Marcel Dekker, New York, 1993: 57-93. 10. Hanlon, G.W., Davies, M.C., Denyer, S.P., Bridgett, J., Bilbruck, J and Martin, G.P. The control of bacterial adhesion by hydrophilic materials - a surface physicochemical approach. In West, R and Batts, G. (eds.), Surface properties of biomaterials, (Proc. Int. Symp. on the Surface Properties of Biomaterials, May 1992, Manchester, UK.), Butterworth-Heinmann Ltd, Oxford, 1994: 71-79. 11. Yianneskis, M., Lee, K.C., Ganderton, D., Marriott, C. and Martin, G.P. Determination of the flow and turbulence characteristics of dry powder inhaler aerosols with laser-doppler anemometry. In Byron, P. and Farr, S. (eds.), Respiratory drug delivery IV, Interpharm Press Inc., Buffalo Grove, Illinois,1994: 99-107. 12. Brown, M.B., Marriott, C. and Martin, G.P. A study of the mechanism of the drug delivery properties of hyaluronan. In: Willoughby, D.A. ed: Second International Workshop on Hyaluronan. Round Table Series No. 36, London: The Royal Society of Medicine Press Ltd., 1995: 29-44. 13. Marriott, C., Martin, G.P. and Brown, M.B. The use of hyaluronan in transdermal drug delivery: physiological barriers and regulatory issues. In: Willoughby, D.A. ed: Third International Workshop on Hyaluronan. Round Table Series No. 40, London: The Royal Society of Medicine Press Ltd., 1995: 38-40. 14. Brown, M.B., Siligardi, G., Martin, G.P., Drake, A. and Marriott, C. An in vitro investigation of the mode of action of hyaluronan as a drug delivery vehicle using circular dichroism. In: Willoughby, D.A. ed: Third International Workshop on Hyaluronan. Round Table Series No. 40, London: The Royal Society of Medicine Press Ltd., 1995: 41-47. 15. Brown, M.B., Ingham, S., Moore, A., Martin, G.P. and Marriott, C. A preliminary study of the effect of hyaluronan on the deposition of 14C-labelled diclofenac within human skin using radiography. In: Willoughby, D.A. ed: Third International Workshop on Hyaluronan. Round Table Series No. 40, London: The Royal Society of Medicine Press Ltd., 1995: 48-52. 16. Brown, M.B., Marriott, C. and Martin, G.P. A study of the transdermal drug delivery properties of hyaluronan. In: Willoughby, D.A. ed: Third International Workshop on Hyaluronan. Round Table Series No. 40, London: The Royal Society of Medicine Press Ltd., 1995: 53-7 17. MacRitchie, H.B., Martin, G.P., Marriott, C. and Murphy, L. Dry powder aerosol characterisation by laser light scattering. Proc. Drug Delivery to the Lungs (London), 6, 118-121, 1995, The Aerosol Society, Bristol (ISBN 0 9512216 8 X) also in summary form in J. Aerosol Med., 9 , 447, 1996 18. Zeng, X.M., Martin, G.P. and Marriott, C. Tetrandrine delivery to the lung: the release characteristics of the drug from albumin microspheres. Proc. Drug Delivery to the Lungs (London), 6, 130-133, 1995, The Aerosol Society, Bristol (ISBN 0 9512216 8 X) also in summary form in J. Aerosol Med., 9 , 448, 1996. 19. Brown, M.B., Bennett, F., Marriott, C. and Martin, G.P. Hyaluronan: a transdermal drug delivery system. An in vitro investigation. Progress in Rheumatology, 6, 59-63, 1996. 20. Brown, M.B., Siligardi, G., Karim, S., Bennett, F.C., Martin, G.P. and Marriott, C. An in vitro study on the effect of hyaluronan on diclofenac binding to human serum albumin. In: Willoughby, D.A. ed: Fourth International Workshop on Hyaluronan. Round Table Series No. 45, London: The Royal Society of Medicine Press Ltd., 1996: 79-85. 21. Bennett, F.C., Brown, M.B., Marriott, C. and Martin, G.P. The diffusion of diclofenac from hyaluronan and other polysaccharide formulations through skin in vitro. In: Willoughby, D.A. ed: Fourth International Workshop on Hyaluronan. Round Table Series No. 45, London: The Royal Society of Medicine Press Ltd., 1996: 86-96. 22. Karim, S., Brown, M.B., Ingham, S., Martin, G.P. and Marriott, C. The determination of the viscosity-average molecular weight of hyaluronan by capilliary viscometry. In: Willoughby, D.A. ed: Fourth International Workshop on Hyaluronan. Round Table Series No. 45, London: The Royal Society of Medicine Press Ltd., 1996: 97-107. 23. Srichana, T., Martin, G.P., Marriott, C. and Swift, D.L. The in vitro assessment of dry powder inhaler formulations: a comparison of drug and carrier deposition on a glass throat model and a human oral cast. Proc. Drug Delivery to the Lungs (London), 7, 32-35, 1996, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 24. Srichana, T., Martin, G.P. and Marriott, C. The effect of device design on drug deposition from dry powder inhaler formulations determined in vitro at different flow rates. Proc. Drug Delivery to the Lungs (London), 7, 36-39, 1996, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 25. Zeng, X.M., Tee, S.K., Martin, G.P. and Marriott, C. Effects of mixing procedure and particle size distribution of carrier particles on the deposition of salbutamol sulphate from dry powder inhaler formulations. Proc. Drug Delivery to the Lungs (London), 7, 40-43, 1996, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 26. MacRitchie, H.B., Martin, G.P., Marriott, C. and Murphy, L. The in vitro characterisation of dry powder blends delivered under in vivo inhalation flow conditions. Proc. Drug Delivery to the Lungs (London), 7, 74-77, 1996, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 27. Abdul-Haq, B., Martin, G.P. and Marriott, C. Drug mucus interactions. Chapter 13 In: Rogers, D.F. and Lethem, M.I. eds: Airway mucus: basic mechanisms and clinical perspectives (respiratory pharmacology and pharmacotherapy), Birkhäuser Verlag, Basel, 1997: 325-337. 28. Srichana, T., Supakovit, T., Martin, G.P. and Marriott, C. The use of hydrophilic poly (ethylene glycol) monodisperse aerosols to calibrate impactors. Proc. Drug Delivery to the Lungs (London), 8, 133-136, 1997, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 29. Srichana, T., Martin, G.P. and Marriott, C. Deposition of lactose and salbutamol from dry powder inhaler blends. Proc. Drug Delivery to the Lungs (London), 8, 103-106, 1997, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 30. Abu Ghoush, A., Zeng, X.M., Martin, G.P., Srichana, T. and Marriott, C. Effects of powder formulation and preparative process on the in vitro deposition of lactose and salbutamol sulphate from dry powder inhalers. Proc. Drug Delivery to the Lungs (London), 8, 163-166, 1997, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 31. Pandal, K.H., Zeng, X.M., Martin, G.P. and Marriott, C. The role of fine particle lactose on the mixing homogeneity and aerodynamic properties of beclomethasone diproprionate from powder formulations for inhalation. Proc. Drug Delivery to the Lungs (London), 9, 72-75, 1998, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 32. Tee, S.K., Zeng, X.M., Martin, G.P. and Marriott, C. The influence of carriers and mixing time on the mixing homogeneity of salbutamol sulphate in dry powder aerosols. Proc. Drug Delivery to the Lungs (London), 9, 188-191, 1998, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 33. Larhrib, H., Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. The use of different grades of lactose as a carrier for aerosolised salbutamol sulphate. Proc. Drug Delivery to the Lungs (London), 9, 192-195, 1998, The Aerosol Society, Bristol (ISBN 0 9529777 0 2). 34. Larhrib, H., Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. Deposition studies of salbutamol sulphate from formulations containing different characterised grades of lactose. In Rubinstein, M. (ed) Proceedings of 18th Pharmaceutical Technology Conference and Exhibition, Utrecht, Volume 2, April 1999: 115-135. 35. Tee, S.K., Martin, G.P., Marriott, C. and Zeng, X.M. The effect of type and concentration of various fine sugar particles on the deposition of salbutamol sulphate from dry powder aerosols in vitro. Proc. Drug Delivery to the Lungs (London), 10, 33-36, 1999, The Aerosol Society, Bristol (ISBN 0 9529777 4 5). 36. Larhrib, H., Martin, G.P., Marriott, C. and Prime, D. The use of engineered lactose crystals as a carrier for aerosolised salbutamol sulphate from dry powder inhalers. Proc. Drug Delivery to the Lungs (London), 10, 155-159, 1999, The Aerosol Society, Bristol (ISBN 0 9529777 4 5). 37. Lansley, A.B. and Martin, G.P. Nasal Drug Delivery. Chapter 9 In: Hillery, A.M. Lloyd, A.W. and Swarbrick, J. eds: Drug Delivery and Targeting for Pharmacists and Pharmaceutical Scientists, Harwood Academic Publishers, Amsterdam, 2000: 237-268. 38. Larhrib, H., Martin, G.P., Marriott, C. and Prime, D. The use of particle engineering to design carriers and drug for respiratory drug delivery. Proc. Drug Delivery to the Lungs (London), 11, 18-21, 2000, The Aerosol Society, Bristol (ISBN 0 9529777 5 3) also in summary form in J. Aerosol Med., 14, 120, 2001. 39. Tee, S.K., Martin, G.P., Leeds, A.R., Walker, C., Kicman, A., Cowan, D.A., Fallon, J. and Marriott, C. In vitro/in vivo evaluation of the performance of two lactose carrier-based dry powder aerosol formulations for the delivery of salbutamol to the lung. Proc. Drug Delivery to the Lungs (London), 11, 176-179, 2000, The Aerosol Society, Bristol (ISBN 0 9529777 5 3) also in summary form in J. Aerosol Med., 14, 130, 2001. 40. Masoud, O., Martin, G.P., Marriott, C. and Nichols, S. The In Vitro Assessment of Aerosolised Drug Deposition Using Novel Oropharyngeal Models. Proc. Drug Delivery to the Lungs (London), 12, 31-34, 2001, The Aerosol Society, Bristol (ISBN 0 9529777 7 X). 41. Tee, S.K., Forbes, B., Larhrib, H., Marriott, C. and Martin, G.P. Development of an in vitro dissolution-absorption model to evaluate the delivery of aerosolised drug from dry powder inhaler formulations. Proc. Drug Delivery to the Lungs (London), 12, 115-118, 2001, The Aerosol Society, Bristol (ISBN 0 9529777 7 X). 42. Al-Najar, M.A., Martin, G.P. and Marriott, C. Poly(lactic-co-glycolic)microspheres as a means of drug delivery to the lung:the effect of process variables on particle size. Proc. Drug Delivery to the Lungs (London), 12, 131-134, 2001, The Aerosol Society, Bristol (ISBN 0 9529777 7 X). 43. Forbes, B., Hashmi, N., Lansley A.B. and Martin, G.P. Adenovirus-mediated gene transfer to respiratory epithelial cells: no inhibition by mucus hypersecretion. Proc. Drug Delivery to the Lungs (London), 12, 175-178, 2001, The Aerosol Society, Bristol (ISBN 0 9529777 7 X). 44. Sajadi Tabassi, A., Martin, G.P. and Marriott, C. The effects of polysorbate surfactants on the structure of mucus glycoprotein. Daru, 5, 6-12, 2001. 45. Sadler, R., Forbes, B., Burnell, P., Prime, D. and Martin, G.P. Modification of the cascade centripeter for delivery of salmeterol to lung cells: theoretical and experimental considerations. Proc. Drug Delivery to the Lungs (London), 13, 23-26, 2002, The Aerosol Society, Bristol (ISBN 0 9529777 9 6). 46. Masoud, O., Martin, G.P., Marriott, C. and Nichols, S. The in vitro assessment of aerosolised drug deposition using novel oropharyngeal models. Proc. Drug Delivery to the Lungs (London), 13, 27-30, 2002, The Aerosol Society, Bristol (ISBN 0 9529777 9 6). 47. Al-Najar, M.A., Martin, G.P. and Marriott, C. Poly (lactic acid) microspheres as a means of drug delivery to the lung: the effect of process variables on particle size. Proc. Drug Delivery to the Lungs (London), 13, 83-86, 2002, The Aerosol Society, Bristol (ISBN 0 9529777 9 6). 48. Liao, Y.H., Brown, M.B., Quader, A. and Martin, G.P. An in vitro investigation of the aerodynamic performance of spray-dried stabilized protein particles. Proc. Drug Delivery to the Lungs (London), 13, 244-247, 2002, The Aerosol Society, Bristol (ISBN 0 9529777 9 6). 49. Brown, M.B., Forbes, B., Hanpanitcharoen, M. and Martin, G.P. The use of hyaluronan in topical drug delivery. In: Kennedy, J. F., Phillips, G.O., Williams P.A. and Hascall V. (Eds): Hyaluronan: biomedical, medical and clinical aspects. Vol II, Woodhead Publishers, Cambridge, 2002 : 249-256. 50. Yff, B.T.S., Royall, P.G., Brown, M.B., Martin, G.P. and Forbes, B. Simulated lung fluid to investigate the biopharmaceutics of inhaled drugs. Proc. Drug Delivery to the Lungs (London), 14, 156-159, 2003, The Aerosol Society, Bristol (ISBN 0 0544567 1 8). 51. Jones, S.A., Martin, G.P. and Brown, M.B. Development of a rapid pre-formulation screen for HFA suspension metered dose inhalers. Respiratory Drug Delivery, 9, 315-317, 2004. 52. Sadler, R., Burnell, P., Prime, D., Smith, C., Martin, G.P. and Forbes, B. In vitro studies into the absorption of inhaled salmeterol xinafoate. Proc. Drug Delivery to the Lungs (London), 15, 162-165, 2004, The Aerosol Society, Portishead (ISBN 0-9544567-3-4). 53. Grainger, C.I., Lockley, D., Martin, G.P. and Forbes, B. Characterisation of an epithelial cell line, Calu-3, as a permeability model of the lung. Proc. Drug Delivery to the Lungs (Edinburgh), 16, 135-138, 2005, The Aerosol Society, Portishead (ISBN 0-9544567-5-0). 54. Grainger, C.I., Greenwell, L.L., Lockley, D.J., Martin, G.P. and Forbes, B. The evaluation of an in vitro depositional system using airway epithelial cells for the permeability of inertially impacted aerosols. Respiratory Drug Delivery, 10, 647-649, 2006, DHI publishing, Illinois (Book 2) (ISBN1-930114-96-6). 55. MacRitchie, H.B., Zeng, X.M., Marriott, C. and Gary Martin, G.P. Assessing Moisture-Sensitivity of Various Dry Powder Aerosol Formulations Using Laser Diffraction and Inertial Impaction. Respiratory Drug Delivery, 10, 651-653, 2006, DHI publishing, Illinois (Book 2) (ISBN1-930114-96-6). 56. Taki, M., Zeng, X.M., Marriott, C. and Gary Martin, G.P. An assessment of the in vitro deposition of aerosolised drugs from a combination Dry powder Inhaler using the Andersen Cascade Impactor (ACI). Respiratory Drug Delivery, 10, 655-658, 2006, DHI publishing, Illinois (Book 2) (ISBN1-930114-96-6). 57. Taki, M., Zeng, X. M., Marriott, C. and Martin, G.P. A comparison of the in vitro deposition profiles of drugs from a combination dry powder inhaler (DPI) using the Next Generation Impactor (NGI). Respiratory Drug Delivery, 10, 659-662, 2006, DHI publishing, Illinois (Book 2) (ISBN1-930114-96-6). 58. Brown, M.B., Jones, S.A., He, W. and Martin, G.P. Hyaluronan- Investigations into the mode of action of hyaluronan in topical delivery. In: Marchessault, R.H., Ravenelle, F. and Zhu, X.X. (Eds.): Polysaccharides in drug delivery and pharmaceutical applications, ACS Press, New York, 2006: 141- 153 (ISBN 978-0-8412-3960-9). 59. Bosquillon, C., Belhadj Salem, L., Hernandez, E., Martin, G.P., Delattre, L., Evrard, B. and Forbes, B. Effect of ß-Cyclodextrin Derivatives on Bronchial Epithelial Cell Layer Permeability Proc. Drug Delivery to the Lungs (Edinburgh), 17, 90-93, 2006, The Aerosol Society, Portishead (ISBN 0-9544567-6-9). 60. Sadler, R., Forbes, B., Burnell, P., Prime, D. and Martin, G.P. Disposition of salmeterol xinafoate particles delivered to lung cells Proc. Drug Delivery to the Lungs (Edinburgh), 17, 248-251, 2006, The Aerosol Society, Portishead (ISBN 0-9544567-6-9). 61. Lim, S.T., Forbes B., Brown, M.B. and Martin, G.P. Physiological factors affecting nasal drug delivery. Chapter 19 In: Touitou, E. and Barry, B.W. (Eds): Enhancement in drug delivery, CRC Press, Boca Raton, 2007: 355- 372 (ISBN 0-8493-3203-6). 62. Brown, M.B., Matthew J. Traynor, M.J., Martin, G.P. and Akomeah, F.K. Transdermal drug delivery systems: skin perturbation devices. Chapter 5 In: Jain, K.K., Methods in Molecular Biology, Vol. 437: Drug Delivery Systems, Humana Press Inc., Totowa, NJ, 2007: 116-136. 63. Taki, M., Marriott, C. and Martin, G.P. Combination formulations for inhalation: benefits and challenges. Proc. Drug Delivery to the Lungs (Edinburgh), 18, 86-89, 2007, The Aerosol Society, Portishead (ISBN 0-9544567-6-9). 64. Murnane, D., Martin, G.P. and Marriott, C. Aerosolisation of inhalable microparticles of fluticasone propionate. Proc. Drug Delivery to the Lungs (Edinburgh), 18, 94-96, 2007, The Aerosol Society, Portishead (ISBN 0-9544567-6-9). 65. Martin, G.P. and Murnane, D. Overcoming barriers to the delivery of inhaled particulate protein pharmaceuticals. In: Faroongsarng, D. and Srichana, T. (eds): Nanotechnology: Application to Drug Delivery Systems, Prince of Songkla University, Thailand, 2008:57-69 (ISBN 978974-11-4932-2). 66. Sadler R.C., Burnell, P.K., Prime, D., Martin G.P. and Forbes, B. Transepithelial flux of salmeterol xinafoate applied as recrystallised or micronised particles to air-interfaced lung cells. Respiratory Drug Delivery, 11, 545-548, 2008 DHI publishing, Illinois (Book 2) (ISBN 1-933722-22-3). 67. Buttini, F., Grainger, C., Jones, S.A., Royall, P.G., Martin, G.P. and Forbes, B. Characterisation of particles emitted from two formulations of beclomethasone dipropionate solution metered dose inhalers. Respiratory Drug Delivery, 11, 581-584, 2008 DHI publishing, Illinois (Book 2) (ISBN 1-933722-22-3). 68. Taki, M., Marriott, C., Zeng X.M. and Martin, G.P. An investigation into the influence of particle size, drug:drug and drug:excipient interactions on the aerodynamic deposition of drugs aerosolised from single and combination dry powder inhalers. Respiratory Drug Delivery, 11, 589-592, 2008 DHI publishing, Illinois (Book 2) (ISBN 1-933722-22-3). 69. Taki, M., Marriott, C., Zeng, X.M. and Martin, G.P. The aerodynamic deposition of drugs from combination DPI formulations: the influence of particle size and drug-drug interactions. Proc. Drug Delivery to the Lungs (Edinburgh), 19, 104-107, 2008, The Aerosol Society, Portishead (ISBN 978-0-9544567-1-9). 70. Murnane, D., Marriott, C. and Martin, G.P. Controlling microcrystalline processes – problems and opportunities. RDD Europe 2009, Vol 1, 167-178, 2009 In; Dalby, R.N., Byron, P.R., Peart, J., Suman, J.D. and Young P.M. eds: Proceedings of Respiratory Drug Delivery Europe, Lisbon, Portugal, Virginia Commonwealth University, Richmond, VA. 71. Jaffari, S., Sandu, G., Martin, G.P., Forbes, B., Collins, E. and Murnane, D. The effect of aerodynamic particle size and solid state disorder on salmeterol xinafoate deposition from pressurized metered dose inhalers and dry powder inhaler formuations. Proc. Drug Delivery to the Lungs (Edinburgh), 2, 222-225, 2011, The Aerosol Society, Portishead (ISBN 978-0-9544567-4-0). In Press 72. Jaffari, S., Collins, E., Khoo, J., Forbes, B., Martin, G.P. and Murnane, D. Aerodynamic size-fractionated sub-populations of salmeterol xinafoate and fluticasone propionate powders possess distinct properties and aerosolisability. Proc. Drug Delivery to the Lungs (Edinburgh), 24, xxx-xxx, 2012, The Aerosol Society, Portishead. 73. Jaffari, S., Collins, E., Murphy, J., Barlow, D., Forbes, B., Martin, G.P. and Murnane, D. Dry dispersion laser diffraction as a generic screening tool for powder deagglomeration. Proc. Drug Delivery to the Lungs (Edinburgh), 24, xxx-xxx, 2012, The Aerosol Society, Portishead. 74. Martin, G.P. and Lansley, A.B. Nasal Drug Delivery. Chapter 38 In Aulton, M.E. and Taylor, K.M.G. (eds): Pharmaceutics – the science of dosage form design (4th Edition), Churchill Livingstone, London, 2013: xxx-xxx. Submitted 75. Jaffari, S., Collins, E., Khoo, J., Forbes, B., Martin, G.P., Murnane, D. Probing the influence of particulate interactions on aerosolisation in combination dry powder formulations. Respiratory Drug Delivery, 13, xxx-xxx, 2012 DHI publishing. (4) Abstracts. 1. Martin, G.P., Marriott, C. and Kellaway, I.W. The effect of natural surfactants on the rheological properties of mucus. J. Pharm. Pharmacol., 28, 76P, 1976. 2. Martin, G.P., Kellaway, I.W. and Marriott, C. The solubilisation and dissolution of progesterone by bile salt mixtures. J. Pharm. Pharmacol., 29, 20P, 1977. 3. Marriott, C., Kellaway, I.W. and Martin, G.P. The effects of bile salts on the physical properties of mucus. Adv. Exp. Med. Biol., 89, 509, 1977. 4. Martin, G.P., Marriott, C. and Kellaway, I.W. The interaction of steroidal hormones with mucus glycoproteins. J. Pharm. Pharmacol., 30, 10P, 1978. 5. Martin, G.P. and Marriott, C. The role of phosphatidylcholine in reducing the toxicity of bile salts to membranes. J. Pharm. Pharm. Pharmacol., 32, 56P, 1980. 6. Jivraj, M., Turner, N.C., Martin, G.P. and Marriott, C. Diffusion of hydrogen ions through gastric mucus. J. Pharm. Pharmacol., 35, 36P, 1983. 7. Wilkins, K.M., Hanlon, G.W., Martin, G.P., Sambee, S.K. and Marriott, C. The role of adhesion in the transmission of bacteria along IUCD polymer threads. J. Pharm. Pharmacol., 35, 51P, 1983. 8. Marriott, C., Martin, G.P., Newbery, R.S. and Turner, N.C. The effect of phosphatidylcholine on bile-salt-induced gastric ulceration in the rat. J. Physiol., 354, 84P, 1984. 9. Jivraj, M., Marriott, C., Martin, G.P. and Turner, N.C. The effect of potentially ulcerogenic agents on hydrogen ion diffusion through native porcine gastric mucus. J. Physiol., 354, 85P, 1984. 10. Brown, D.T., Topham, J.D. and Martin, G.P. A rational approach to the official B.P. test for the determination of yield values of viscoelastic gels. J. Pharm. Pharmacol., 36, 20P, 1984. 11. Newbery, R.S., Turner, N.C., Martin, G.P. and Marriott, C. Gastric ulceration: The effects of bile salt and biliary phospholipid in the rat. J. Pharm. Pharmacol., 36, 44P, 1984. 12. Lam, T.P., Martin, G.P., Bell, A.E. and Marriott, C. The effect of humidity on the particle size distribution of salbutamol measured over realistic deposition time intervals. J. Pharm. Pharmacol., 36, 84P, 1984. 13. Wilkins, K.M., Hanlon, G.W., Martin, G.P. and Marriott, C. The influence of surface free energy and microrugosity on the adhesion of bacteria to polymer monofilaments. J. Pharm. Pharmacol., 37, 65P, 1985. 14. Wilkins, K.M., Hanlon, G.W., Martin, G.P. and Marriott, C. Factors influencing the transmission of bacteria along polymeric threads. J. Pharm. Pharmacol., 37, 66P, 1985. 15. Newbery, R.S., Martin, G.P., Turner, N.C. and Marriott, C. The effects of lysophosphatidylcholine on the rat gastric mucosa at acid and neutral pH. J. Pharm. Pharmacol., 37, 101P, 1985. 16. Patel, U.B., Bell, A.E., Martin, G.P. and Marriott, C. An in vitro comparison of drug deposition from a commercially available dry powder inhaler with a pressurised aerosol pack. J. Pharm. Pharmacol., 37, 108P, 1985. 17. Newbery, R.S., Martin, G.P., Turner, N.C. and Marriott, C. Mucosal injury: the combined effects of lysophosphatidylcholine and sodium taurodeoxycholate on the rat stomach. J. Pharm. Pharmacol., 37, 144P, 1985. 18. Newbery, R.S., Martin, G.P. and Marriott, C. The effect of bile salt and phosphatidylcholine on the rheological properties of pig purified gastric mucus. J. Pharm. Pharmacol., 38, 55P, 1986. 19. Jacobs, M., Martin, G.P. and Marriott, C. Effects of phosphatidylcholine on topical corticosteroid bioavailability in vivo. J. Pharm. Pharmacol., 38, 69P, 1986. 20. Newbery, R.S., Martin, G.P., Loveday, B.E. and Machling, R.C. An assessment of the influence of certain bile components upon acute gastric mucosal damage induced in the conscious rat. J. Pharm. Pharmacol., 38, 120P, 1986. 21. Cutler, L.M., Martin, G.P., Newbery, R.S. and Marriott, C. The relative membrane toxicity of bile salts found in man. J. Pharm. Pharmacol., 39, 29P, 1987. 22. Batts, A.H., Marriott, C., Martin, G.P. and Bond, S.W. The influence of preservatives on mucociliary transport rate in the frog palate. J. Pharm. Pharmacol., 39, 92P, 1987. 23. O'Grady, D.M., Newbery, R.S., Martin, G.P. and Marriott, C. The effect of tri-potassium di-citrato bismuthate on hydrogen ion diffusion across native porcine gastric mucus. J. Pharm. Pharmacol., 39, 93P, 1987. 24. Jacobs, M., Martin, G.P. and Marriott, C. A comparison of corticosteroid bioavailability from semi-solid and liposome formulations. J. Pharm.Pharmacol., 39, 142P, 1987. 25. Marriott, C. and Martin, G.P. The effect of sodium thiophene carboxylate on the properties of pig tracheal mucus. Thorax, 43, 256P, 1988. 26. El-Hariri, L.M., Martin, G.P., Marriott, C. The effect of pH on human membrane pertubation by bile salts found in man. J. Pharm. Pharmacol., 40, 13P, 1988. 27. Martin, G.P., Marriott, C. and Loveday, B.E. The in-vivo effect of oxytetracycline on the rheological properties of tracheobronchial mucus from the mini-pig. J. Pharm. Pharmacol., 40, 94P, 1988. 28. Martin, G.P., Marriott, C. and Fairbrother, J.E. The use of hydrocolloid dressings in topical bioavailability studies in man. J. Pharm. Pharmacol., 40, 104P, 1988. 29. Batts, A.H., Marriott, C., Martin, G.P. and Bond, S.W. Do pharmaceutical preservatives influence mucociliary clearance? Eur. Resp. J., 1 (suppl. 1) 104s, 1988. 30. El-Hariri, L.M., Martin, G.P. and Marriott, C. Membrane disruption by bile salts: a comparison of the protective effect of some phospholipids. J. Pharm. Pharmacol., 41, 74P, 1989. 31. El-Hariri, L.M., Martin, G.P. and Marriott C. The effect of sodium taurodeoxycholate on the rheological properties of pig native and purified gastric mucus. J.Pharm.Pharmacol., 41, 75P, 1989. 32. Helliwell, M., Martin, G.P. and Marriott, C. The release of arachis oil from gelatin-acacia microcapsules. J. Pharm. Pharmacol., 41, 117P, 1989. 33. Martin, G.P., Hollingsbee, D.A. and Marriott, C. The effect of dose under a hydrocolloid patch on the human bioavailability of a topical corticosteroid. J. Pharm. Pharmacol., 41, 129P, 1989. 34. Townes, A.B., Martin, G.P., James, S.L. and Marriott, C. Characterisation of microparticulate surface groups. J. Pharm. Pharmacol., 41, 140P, 1989. 35. El-Hariri, L.M., Martin, G.P., Olliff, C.J. and Marriott, C. Development of a high performance liquid chromatography assay to separate conjugated and unconjugated bile salts. J. Pharm. Pharmacol., 41, 146P, 1989. 36. El-Hariri, L., Martin, G.P. and Marriott, C. The effect of sodium taurodeoxycholate on the rheological properties of native gastric mucus. Biorheology, 26, 616, 1989. 37. King, A.J., Tukker, J.J., Marriott, C., Martin, G.P. and Taylor, D.C. The effects of carbenoxolone and cimetidine on mucus output in the rat ileum. J. Pharm. Pharmacol., 42, 36P, 1990. 38. Bilbruck, J., Hanlon, G.W. and Martin, G.P. Bacterial adhesion to polyhema coated monofilament fibres. J. Pharm. Pharmacol., 42, 65P, 1990. 39. Bilbruck, J., Hanlon, G.W. and Martin, G.P. Modification of the surface characteristics of polymer monofilaments by hydrogel coating. J. Pharm. Pharmacol., 42, 100P, 1990. 40. Helliwell, M., Martin, G.P. and Marriott, C. The effect of temperature and time on the in-vitro adhesion of putative mucoadhesives to mucus. J. Pharm. Pharmacol., 42, 130P, 1990. 41. Shafi, Z.B., Martin, G.P. and James, S.L. Factors affecting high shear preparation of albumin microspheres. J. Pharm. Pharmacol., 42, 144P, 1990. 42. Helliwell, M., Martin, G.P. and Marriott, C. The characterisation of surface groups on gelatin-acacia microcapsules. J. Pharm. Pharmacol., 42, 148P, 1990. 43. Ladenheim, D., Martin, G.P., Marriott, C. and Hollingsbee, D.A. In-vivo hydration of a hydrocolloid containing dermatological patch and its effect on transepidermal water loss. J. Pharm. Pharmacol., 42, 158P, 1990. 44. Ladenheim, D., Martin, G.P., Marriott, C. and Hollingsbee, D.A. Hydration of a hydrocolloid containing dermatological patch and its effects on rheological characteristics. J. Pharm. Pharmacol., 42, 159P, 1990. 45. Hollingsbee, D.A., Martin, G.P., Walker, M., Marriott, C. and Fairbrother, J. The effect of semi-permeable film covers on the bioavailability of betamethasone valerate delivered from a topical cream vehicle. J. Pharm. Pharmacol., 42, 160P, 1990. 46. Allen, J.D., Martin, G.P. and Marriott, C. Growth and characterisation of a human colonic mucin secreting cell line HT29-18N2. J. Pharm. Pharmacol., 42, 163P, 1990. 47. King, A.J., Tukker, J.J., Marriott, C., Martin, G.P. and Taylor, D.C. The effects of glycine and methacholine on mucus output in the rat ileum. J. Pharm. Pharmacol., 42, 180P, 1990. 48. Hollingsbee, D.A., Fairbrother, J.E., Martin, G.P. and Marriott, C. The use of alternative methods for conducting skin blanching (vasoconstriction) studies with topical corticosteroid products. Pharm. Res., 7, S50, 1990. 49. Hollingsbee, D.A., Martin, G.P., Marriott, C. and Fairbrother, J.E. The influence of hydrocolloid containing dermatological patch on the skin blanching (vasoconstriction) response induced by triamcinolone acetonide cream. Pharm. Res., 7, S50, 1990. 50. Waring, M.J., Monger, L., Hollingsbee, D.A., Martin, G.P. and Marriott, C. Evaluation of the Minolta Chromometer CR200 for the assessment of steroid induced blanching. Pharm. Res., 7, S50, 1990. 51. Bilbruck, J., Hanlon, G.W. and Martin, G. P. Investigation of the effects of transcervical monofilament insertion on the microbial status of the uterus in guinea pigs. J. Pharm. Pharmacol., 43, 13P, 1991. 52. Helliwell, M., Martin, G.P. and Marriott, C. The preparation of microcapsule - poly(acrylic acid) conjugates and assessment of in-vitro mucoadhesion. J. Pharm. Pharmacol., 43, 27P, 1991. 53. Ladenheim, D., Monger, L., Martin, G.P., Marriott, C. and Hollingsbee, D. The effect of hydrocolloid composition on hydration and corticosteroid induced blanching of a dermatological patch. J. Pharm. Pharmacol., 43, 54P, 1991. 54. Monger, L., Martin, G.P., Marriott, C., Hollingsbee, D. and Fairbrother, J.E. The effect of dose and application time on the topical bioavailability of triamcinolone acetonide delivered from a hydrocolloid-containing patch. J. Pharm. Pharmacol., 43, 57P, 1991. 55. Ladenheim, D., Edwardson, P.A.D., Walker, M., Martin, G.P., Marriott, C. and Lloyd, A.W. The assessment of skin hydration produced by occlusion using fourier transform infrared spectroscopy. J. Pharm. Pharmacol., 43, 58P, 1991. 56. Hastewell, J., Phillips, J., Lloyd, A.W., Martin, G.P., Marriott, C. and Williams, M. The evaluation of microbially activated colonic drug delivery systems. J. Pharm. Pharmacol., 43, 61P, 1991. 57. MacAdam, A., Martin, G.P., James, S.L. and Marriott, C. Preparation and characterisation of anti-porcine gastric mucus antibodies. J. Pharm. Pharmacol., 43, 62P, 1991. 58. Allen, J.D., Martin, G.P., Marriott, C., Hassan I. and Williamson, I. Drug transport across a novel mucin secreting cell model: comparison with the Caco-2 cell system. J. Pharm. Pharmacol., 43, 63P, 1991. 59. King, A.J., Martin, G.P. and Marriott, C. The effects of amino acids on mucus output in the rat intestine. J. Pharm. Pharmacol., 43, 64P, 1991. 60. Bilbruck, J., Hanlon, G.W. and Martin, G.P. Effect of mucus on the bactericidal activity of chlorhexidine-impregnated monofilaments. J. Pharm. Pharmacol., 43, 133P, 1991. 61. King, A.J., Martin, G.P., Marriott, C. and Evans, R. Effects of amino acids on intestinal mucus output in the rat. Proc. Int. Symp. Control. Rel. Bioact. Mater., 18, 566-567, 1991 62. Helliwell, M., Martin, G.P. and Marriott, C. The in situ mucoadhesion of microcapsule-polymer conjugates. Proc. Int. Symp. Control. Rel. Bioact. Mater., 18, 572-573, 1991. 63. Allen, J.D., Lowe, P., Martin, G.P. and Marriott, C. Use of confocal microscopy to characterise Caco-2 and Ht29GlucH cell populations in a coculture drug transport model. J. Pharm. Pharmacol., 44, 1064, 1992. 64. Shafi, Z.B., Martin, G.P., Olliff, C.J. and James, S.L. The tissue distribution of albumin microspheres after oral administration to rats. J. Pharm. Pharmacol., 44, 1084, 1992. 65. MacAdam, A.B., James, S.L., Martin, G.P. and Marriott, C. The mucoadhesive properties of antibody-coated bovine serum albumin microspheres. J. Pharm. Pharmacol., 44, 1085, 1992. 66. King, A.J., Martin, G.P. and Marriott, C. The effects of frusemide on glycine-induced mucus output in the rat intestine. J. Pharm. Pharmacol., 44, 1095, 1992. 67. Lloyd, A.W., Soozandehfar, S.H., Martin, G.P., Marriott, C., Williams, M., Hastewell, J. and Phillips, J. Studies of azopolymer based colonic drug delivery systems. Proc. 1st Ann. Conf. UKaps (York), Hadgraft J, Kellaway, I.W. and Parr, G. D. (eds), STS publishing, Cardiff, p.21, 1992. 68. Ladenheim, D., Monger, L.S., Martin, G.P., Marriott, C. and Hollingsbee, D.A. Enhanced percutaneous penetration of triamcinolone acetonide using a hydrocolloidcontaining dermatological patch. Proc. 1st Ann. Conf. UKAPS (York), Hadgraft J, Kellaway, I.W. and Parr, G. D. (eds), STS publishing (ISBN 0 948917 02 4), Cardiff, p.49, 1992. 69. MacAdam, A.B., Martin, G.P., James, S.L. and Marriott, C. Drug targeting in the gastrointestinal tract. Proc. Int. Symp. Control. Rel. Bioact. Mater., 19, 277-278, 1992. 70. Ladenheim, D., Monger, L.S., Lloyd, A.W., Martin, G.P., Marriott, C., Edwardson, P.A.D. and Hollingsbee, D.A. The effect of a dermatological patch on stratum corneum hydration and percutaneous absorption. Proc. Int. Symp. Control. Rel. Bioact. Mater., 19, 460-461, 1992. 71. Shafi, Z.B., Martin, G.P., Olliff, C.J. and James, S.L. The in vivo distribution of albumin microspheres after oral administration in rats. Proc. Int. Symp. Control. Rel. Bioact. Mater., 19, 506-507, 1992. 72. Martin, G.P., El-Hariri, L.M. and Marriott, C. Effect of pH on sodium taurodeoxycholate-induced changes to the rheological properties of mucus. Biorheology, 29, 156, 1992. 73. Timsina, M.P., Martin, G.P., Marriott, C., Lee, K.C., Suen, K.O. and Yianneskis, M. Studies on the measurement of peak inspiratory flow rate (PIF) with dry powder inhaler devices in healthy volunteers. Thorax, 48, 433, 1993. 74. Lloyd, A.W., Hodges, N.A., Soozandehfar, S.H. and Martin, G.P. Azo reduction is mediated by enzymatically generated low molecular weight electron carriers. J. Pharm. Pharmacol., 45, 1107, 1993. 75. Heer, J.S., Martin, G.P. and Marriott, C. The effect of bile salts on the rheological properties of mucus gels of different purities. J. Pharm. Pharmacol., 45, 1109, 1993. 76. Bragger, J., Lloyd, A.W., Barlow, D., Martin, G.P., Bloomfield, S.F., Phillips, J. Application of molecular modelling to the design of azo compounds for use in colon specific drug delivery. J. Pharm. Pharmacol., 45, 1126, 1993. 77. Gould, L.A., Cook, J.M. and Martin, G.P. The haemolytic potential of mixtures of egg phophatidylcholine and polyoxyethylene ether surfactants. J. Pharm. Pharmacol., 45, 1132, 1993. 78. Pritchard, K., Lansley, A.H. and Martin, G.P. The effects of sodium deoxycholate and egg phosphatidylcholine on mucociliary transport rate. J. Pharm. Pharmacol., 45, 1133, 1993. 79. Bragger, J., Lloyd, A.W., Marriott, C. Bloomfield, S.F., Martin, G.P. and Phillips, J. Bacterial cleavage of azo compounds to free amines. J. Pharm. Pharmacol., 45, 1134, 1993. 80. Haghpanah, M., Martin, G.P. and Marriott, C. Albumin microspheres as a means of controlled drug delivery to the airway. J. Pharm. Pharmacol., 45, 1138, 1993. 81. Timsina, M., Martin, G.P., Marriott, C., Ganderton, D., Lee, K.C., Suen, K.O. and Yianneskis, M. Studies on the effect of dry powder inhalers upon peak inspiratory flow (PIF) in volunteers. J. Aerosol Med., 6 (Supplement), 41, 1993. 82. Lee, K.C., Suen, K.O., Yianneskis, M., Marriott, C., Martin, G.P., Timsina, M. and Ganderton, D. Flow characteristics of dry powder inhalers. J. Aerosol Med., 6 (Supplement), 41, 1993. 83. Haghpanah, M., Marriott, C. and Martin, G.P. The use of albumin microspheres in respiratory drug delivery. J. Aerosol Med., 6 (Supplement), 54, 1993. 84. Soozandehfar, S.H., Baker, J.A. Lloyd, A.W. and Martin, G.P. The synthesis of p, p'-divinylazobenzene for fabrication of azopolymer devices for colon specific drug delivery. Proc. 2nd Ann. Conf. UKaps (Exeter), Hadgraft, J., Kellaway, I.W. and Parr, G.D. (eds), STS publishing (ISBN 0 948917 05 9), Cardiff, p.40, 1993. 85. Bragger, J., Lloyd, A.W., Marriott, C. Bloomfield, S.F., Martin, G.P. and Phillips, J. Redox potential as a factor influencing azo-reduction. Proc. 2nd Ann. Conf. UKaps (Exeter), Hadgraft, J., Kellaway, I.W. and Parr, G.D. (eds), STS publishing (ISBN 0 948917 05 9), Cardiff, p.56, 1993. 86. Gould, L.A., Cook, J.M. and Martin, G.P. Membrane disruption by polyoxyethylene ether surfactants: the influence of phosphatidylcholine. Proc. 2nd Ann. Conf. UKaps (Exeter), Hadgraft, J., Kellaway, I.W. and Parr, G.D. (eds), STS publishing (ISBN 0 948917 05 9), Cardiff, p.69, 1993. 87. Lee, K.C., Suen, K.O., Yianneskis, M., Marriott, C., Martin, G.P., Timsina, M. and Ganderton, D. Turbulence generation in dry powder inhalers. Proc. 2nd Ann. Conf. UKaps (Exeter), Hadgraft, J., Kellaway, I.W. and Parr, G.D. (eds), STS publishing (ISBN 0 948917 05 9), Cardiff, p.73, 1993. 88. Haghpanah, M., Martin, G.P. and Marriott, C. Albumin microspheres as pulmonary delivery systems: an in vitro study. Proc. 2nd Ann. Conf. UKaps (Exeter), Hadgraft, J., Kellaway, I.W. and Parr, G.D. (eds), STS publishing, Cardiff, p.74, 1993. 89. Shah, P.L., Scott, S., Lansley, A., Martin, G.P., Marriott, C. and Hodson, M.E. Preliminary report on in vivo reduction of sputum viscoelasticity in cystic fibrosis patients treated with aerosolised recombinant human DNase. Thorax, 49, 394P, 1994. 90. Brown, M.B., Martin, G.P. and Marriott, C. An in vitro investigation of the interaction of diclofenac with hyaluronan. J. Pharm. Pharmacol., 46, 1049, 1994. 91. Sajadi Tabassi, S.A., Marriott, C. and Martin, G.P. The haemolytic effects of nonionic surfactants. J. Pharm. Pharmacol., 46, 1060, 1994. 92. Abdul-Haq, B., Marriott, C. and Martin, G.P. The effect of ATP, ADP and ADP on intestinal goblet cell mucin secretion. J. Pharm. Pharmacol., 46, 1068, 1994. 93. Pritchard, K., Lansley, A.H. and Martin, G.P. The effects of sodium taurodihydrofusidate on mucus glycoprotein secretion by excised rat tracheae. J. Pharm. Pharmacol., 46, 1070, 1994. 94. Gould, L.A., Lansley, A.H. and Martin, G.P. Penetration enhancement by sodium dodecyl sulphate and polyoxyethylene 10 lauryl ether: the effect of phospholipid. J. Pharm. Pharmacol., 46, 1074, 1994. 95. Haghpanah, M., Marriott, C. and Martin, G.P. Drug delivery to the lung using albumin microspheres. J. Pharm. Pharmacol., 46, 1075, 1994. 96. Malhi, S.K., Younus, I.Y., James, S.L., Martin, G.P. and Malhi, J.S. Development of an assay to quantify microsphere-bound lectin. J. Pharm. Pharmacol., 46, 1086, 1994. 97. Gould, L.A., Lansley, A.H. and Martin, G.P. The effects of egg phosphatidylcholine on the penetration enhancing effects of Brij 96. Proc. 3rd Ann. Conf. UKaps (Leicester), Hadgraft, J., Kellaway, I.W. and Parr, G.D. (eds), STS publishing (ISBN 0 948917 05 9), Cardiff, p.44, 1994. 98. Pritchard, K., Lansley, A.H. and Martin, G.P. The effects of sodium taurodihydrofusidate and egg phosphatidylcholine on mucociliary transport rate. Proc. 3rd Ann. Conf. UKaps (Leicester), Hadgraft, J., Kellaway, I.W. and Parr, G.D. (eds), STS publishing (ISBN 0 948917 07 5), Cardiff, p.50, 1994. 99. Bragger, J., Lloyd, A.W., Marriott, C., Bloomfield, S.F., Martin, G.P. and Phillips, J. Investigations into the reduction of a polymeric cross-linking compound, 4,4'-dihydoxyazobenzene, by the colonic bacterium B. fragilis. Proc. 3rd Ann. Conf. UKaps (Leicester), Hadgraft, J., Kellaway, I.W. and Parr, G.D. (eds), STS publishing (ISBN 0 948917 07 5), Cardiff, p.78, 1994. 100. Soozandehfar, S.H., Bragger, J., Lloyd, A.W., Baker,J., Barlow, D., Martin, G.P., Marriott, C., Bloomfield, S.F. and Phillips, J. Investigating methods of predicting the ease of reduction of synthetic azo compounds. Proc. 3rd Ann. Conf. UKaps (Leicester), Hadgraft, J., Kellaway, I.W. and Parr, G.D. (eds), STS publishing (ISBN 0 948917 07 5), Cardiff, p.81, 1994. 101. Bragger, J., Lloyd, A.W., Marriott, C., Bloomfield, S.F., Martin, G.P. and Phillips, J. Some factors influencing the azo reductase activity of B. fragilis. Proc. Int. Symp. Control. Rel. Bioact. Mater., 21, 256-257, 1994. 102. Gould, L.A., Lansley, A.H. and Martin, G.P. Phospholipid and surfactant mixed aggregates as penetration enhancers. Proc. Int. Symp. Control. Rel. Bioact. Mater., 21,573-574, 1994. 103. MacRitchie, H.B., Martin, G.P., Marriott, C. and Murphy, L. Determination of the inspirable properties of dry powder aerosols using laser light scattering. J. Pharm. Pharmacol., 47, 1072, 1995. 104. Olliver, M.P., Lansley, A.B. and Martin, G.P. The characterisation of a gene delivery system comprising DNA and cationic liposomes. J. Pharm. Pharmacol., 47, 1083, 1995. 105. Soozandehfar, S.H., Lloyd, A.W., Hodges, N.A. and Martin, G.P. Investigations into the mechanism of biological azo reduction. J. Pharm. Pharmacol., 47, 1120, 1995. 106. Brown, M.B., Martin, G.P. and Marriott, C. An in vitro investigation of the transdermal drug delivery properties of hyaluronan. J. Pharm. Pharmacol., 47, 1122, 1995. 107. Bragger, J., Lloyd, A.W., Marriott, C., Bloomfield, S.F., Martin, G.P. and Phillips, J. Bacterial cleavage of novel azo polymer cross-linking compounds to free amines. J. Pharm. Pharmacol., 47, 1122, 1995. 108. Brown, M.B., Siligardi, G., Martin, G.P., Marriott, C. and Drake, A. A study of the effect of hyaluronan on the binding of diclofenac to serum albumin using circular dichroism. J. Pharm. Pharmacol., 47, 1123, 1995. 109. Shiralizadeh, F., Marriott, C. and Martin, G.P. The effect of various buffering systems on the solubility of silver sulphadiazine. J. Pharm. Pharmacol., 47, 1125, 1995. 110. Heer, J.S., Roberts, C.J. Davies, M.C., Martin, G.P. and Marriott, C. The effect of bile salts on the structure of mucus. J. Pharm. Pharmacol., 47, 1125, 1995. 111. Sajadi Tabassi, S.A., Marriott, C. and Martin, G.P. The effect of aliphatic mercaptans on the rheological properties of mucus gel. J. Pharm. Pharmacol., 47, 1127, 1995. 112. Pritchard, K., Lansley, A.H. and Martin, G.P. The effects of sodium taurodihydrofusidate on mucociliary transport rate and mucus glycoprotein secretion using two ex-vivo animal models. Amer. Resp. Crit. Care Med., 151, A263, 1995. 113. Sajadi Tabassi, S.A., Martin, G.P. and Marriott, C. Membrane disruption by some pharmaceutical excipients. Proc. 4th Ann. Conf. UKaps (Nottingham), Kellaway, I.W. and Hadgraft, J. (eds), STS publishing (ISBN 0 948917 08 3), Cardiff, p.73, 1995. 114. MacRitchie, H. B., Martin, G.P., Marriott, C. and Murphy, L. The particle characterisation of dry powder aerosols using laser light scattering. J. Aerosol Med., 8, 102, 1995. 115. Soozandehfar, S.H., Lloyd, A.W., Olliff, C.J., Hodges, N.A. and Martin,G.P. A polarographic method for the assessment of azo reduction by low-molecular-weight electron carriers. Proc. Int. Symp. Control. Rel. Bioact. Mater., 22, 238-239, 1995. 116. MacRitchie, H.B., Martin, G.P., Marriott, C. and Murphy, L. Dry powder aerosol characterisation by laser light scattering. J. Aerosol Med., 9, 447, 1996. 117. Zeng, X.M., Martin, G.P. and Marriott, C. Tetrandrine delivery to the lung: the release characteristics of the drug from albumin microspheres. J. Aerosol Med., 9, 448, 1996. 118. Pritchard, K., Lansley, A.H. and Martin, G.P. The effects of sodium taurodihydrofusidate and egg phosphatidylcholine (PC) on mucus glycoprotein secretion of excised rat tracheae. Amer. Resp. Crit. Care Med., 153, A854, 1996. 119. Srichana, T., Martin, G.P. and Marriott, C. The in vitro deposition of lactose carrier from dry powder inhaler formulations. Thorax, 51 (Supplement 3), A74 (P194), 1996. 120. Zeng, X.M., Tee, S.K., Martin, G.P. and Marriott, C. Improving the delivery efficiency of dry powder inhalers (DPIs) by adding fine carrier particles to powder formulations. Thorax, 51 (Supplement 3), A74 (P195), 1996. 121. MacRitchie, H.B., Martin, G.P., Marriott, C. and Murphy, L. Dry powder aerosol from Bricanyl Turbohalers® measured by inertial impaction and laser light scattering at different flow rates. Eur. J. Pharm. Sci., 4 (Suppl.), S142 (P.2.046), 1996. 122. Siligardi, G., Brown, M.B., Karim, S., Bennett, F.C., Martin, G.P., Drake, A. and Marriott, C. Investigation of the effect of hyaluronan on drug-protein binding using circular dichroism. Eur. J. Pharm. Sci., 4 (Suppl.), S164 (P.3.007), 1996. 123. Karim, S., Brown, M.B., Ingham, S., Martin, G.P. and Marriott, C. Rheological and molecular weight studies of hyaluronan. Eur. J. Pharm. Sci., 4 (Suppl.), S175 (P.3.042), 1996. 124. Crooks, J., Marriott, C., Martin, G.P., Paget, T. and Hassal, D. Adhesive interactions between cell membranes. Eur. J. Pharm. Sci., 4 (Suppl.), S195 (P.3.109), 1996. 125. Siligardi, G., Brown, M.B., Martin, G.P, Karim, S. and Marriott, C. The effect of hyaluronan on diclofenac binding to albumin. Pharm. Res., 13 (Suppl.), S-256 (PDD 7094), 1996. 126. Crooks, J., Marriott, C., Martin, G.P., Paget, T. and Hassall, D. Lectin type adhesion between isolated membranes. Pharm. Res., 13 (Suppl.), S-283 (PDD 7203), 1996. 127. Brown, M.B., Martin, G.P., Bennett, F. and Marriott, C. Franz cell studies of hyaluronan as a drug delivery agent. Pharm. Res., 13 (Suppl.), S-374 (PDD 7568), 1996. 128. Karim, S., Brown, M.B., Ingham, S., Martin, G.P., Marriott, C. Rheological and viscosity-average molecular weight studies of hyaluronan. British Soc. Rheol. Bull., 39, 80, 1996 (ISSN-0045-3145). 129. Pritchard, K., Lansley, A.B. and Martin, G.P. Phosphatidylcholine (PC) mitigates effects of sodium taurodihydrofusidate (STDHF) on frog ciliary bear frequency. Amer. Resp. Crit. Care Med., 155, A778, 1997. 130. Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. Effects of crystallisation conditions on the morphology of lactose intended for use as a carrier for dry powder inhalers. J. Pharm. Pharmacol., 49 (Suppl. 4), 24 (abstract 37), 1997. 131. Sajadi Tabassi, S.A., Marriott, C. and Martin, G.P. The effect of polysorbates on the rheological properties of mucus gel. J. Pharm. Pharmacol., 49 (Suppl. 4), 38 (abstract 60), 1997. 132. Gould, L.A., Lawrence, M.J., Lansley, A.B. and Martin, G.P. Membrane disruption induced by phospholipid and surfactant mixed aggregates:the influence of particle size and aggregate type. J. Pharm. Pharmacol., 49 (Suppl. 4), 45 (abstract 75), 1997. 133. Srichana,T., Martin, G.P. and Marriott, C. The effect of a modified mouthpiece on drug delivery from dry powder inhalers. J. Pharm. Pharmacol., 49 (Suppl. 4), 69 (abstract 122), 1997. 134. Pritchard, K., Lansley, A.B. and Martin, G.P. The effects of sodium taurodihydrofusidate and phosphatidylcholine on ciliary beat frequency in vitro. J. Pharm. Pharmacol., 49 (Suppl. 4), 85 (abstract 154), 1997. 135. Nazir, T., Gould, L.A., Marriott, C., Martin, G.P. and Brown, M.B. Improved chromatography of cyclosporin A using a porous graphitic carbon stationary phase. J. Pharm. Pharmacol., 49 (Suppl. 4), 90 (abstract 164), 1997. 136. Karim, S., Brown, M.B., Ingham, S., Martin, G.P. and Marriott, C. A rheological study including the effect of heat on the viscosity-average molecular weight of the drug targeting agent hyaluronan. Pharm. Res., 14 (Suppl.), S-45 (1143), 1997. 137. Gould, L.A., Brown, M.B., Martin, G.P. and Marriott, C. Hyaluronan antioxidant activity within liposomal systems. Pharm. Res., 14 (Suppl.), S-45 - S-46 (1144), 1997. 138. Bennett, F.C., Brown, M.B., Martin, G.P. and Marriott, C. Intradermal localisation of diclofenac using a hyaluronan-containing topical vehicle. Pharm. Res., 14 (Suppl.), S-116 (1338), 1997. 139. Zeng, X.M., Martin, G.P., Marriott, C. and Pritchard, J. Effects of surface smoothness of lactose on the delivery of salbutamol sulphate from dry powder inhalers. Pharm. Res., 14 (Suppl.), S-136 - S-137 (1385), 1997. 140. Srichana, T., Martin, G.P. and Marriott, C. The effect of an oral cast on the lower stage deposition of dry powder inhaler formulations in a twin stage impinger. Pharm. Res., 14 (Suppl.), S-141 - S-142 (1402), 1997. 141. Nazir, T., Gould, L.A., Marriott, C., Martin, G.P. and Brown, M.B. Improved chromatography of cyclosporin analogues using a highly temperature stable porous graphitic carbon stationary phase. Pharm. Res., 14 (Suppl.), S-569 - S-570 (3324), 1997. 142. Rao, A., Desai, J.B., Bayliss M., Martin, G.P. and Lansley, A.B. Investigation of the absorption characteristics of 5HT1 agonists using the 16HBE14o- cell culture model of the airway epithelium. Pharm. Res., 14 (Suppl.), S-634 (4006), 1997. 143. Zeng, X.M., Martin, G.P. and Marriott, C. The effect of molecular weight of polyvinylpyrrolidone on the cystallisation of colyophilized sucrose. J. Pharm. Pharmacol., 50 (Suppl.), 65, 1998. 144. Srichana, T., Martin, G.P., Kicman, A., Walker, C. and Marriott, C. Determination of salbutamol in human plasma and urine by gas chromatography-mass spectrometry. J. Pharm. Pharmacol., 50 (Suppl.), 86, 1998. 145. Rao, A., Martin, G.P. and Lansley, A.B. Transport of a series of D-oligopeptides across cultured 16HBE14o- and alveolar cells. J. Pharm. Pharmacol., 50 (Suppl.), 137, 1998. 146. Hanpanitcharoen, M., Brown, M.B. and Martin, G.P. The role of glycosaminoglycans on the skin partioning of ibuprofen. J. Pharm. Pharmacol., 50 (Suppl.), 183, 1998. 147. Karim, S., Brown, M.B., Bennett, F.C., Martin, G.P. and Marriott, C. The effect of hyaluronan on the binding of diclofenac to human albumin using equilibrium dialysis. J. Pharm. Pharmacol., 50 (Suppl.), 197, 1998. 148. Srichana, T., Brain, A., Martin, G.P. and Marriott, C. The determination of drug-carrier interactions in dry powder inhaler formulations. J. Aerosol Sci., 29 (Suppl. 1), S757-S758, 1998. 149. Srichana, T., Martin, G.P. and Marriott, C. Calibration method for the Andersen cascade impactor. J. Aerosol Sci., 29 (Suppl. 1), S761-S762, 1998. 150. Rao, A., Martin, G.P. and Lansley, A.B. The absorption characteristics of 5HT1 agonists across airway and alveolar cells grown in culture. Thorax, 53 (Suppl. 4), A36 (abstract P50), 1998. 151. Hashmi, N., Lansley, A.B., Martin, G.P. and Forbes, B. Effect of purinergic stimulation on mucus secretion and barrier properties of cultured airway epithelial cells (SPOC1). Resp. Med., 92, A.21-A.22, 1998. 152. Martin, G.P. The development of polymers for targeted delivery of drugs to the colon. Eur. J. Pharm. Sci., 6 (Suppl. 1), S14 (Abstract 55), 1998. 153. Srichana, T., Martin, G.P. and Marriott, C. Enantiomeric separation of salbutamol in urine. Eur. J. Pharm. Sci., 6 (Suppl. 1), S44 (Abstract 174), 1998. 154. Hanpanitcharoen, M., Brown, M.B. and Martin, G.P. The effect of glycosaminoglycans on the skin partioning of diclofenac. Eur. J. Pharm. Sci., 6 (Suppl. 1), S62 (Abstract 245), 1998. 155. Karim, S., Brown, M.B., Bennet, F.C., Martin, G.P. and Marriott, C. The effect of hyaluronan on the binding of diclofenac to human albumin: equilibrium dialysis studies. Eur. J. Pharm. Sci., 6 (Suppl. 1), S62 (Abstract 246), 1998. 156. Rao, A., Martin, G.P. and Lansley, A.B. Absorption characteristics of salmeterol across Caco-2, 16HBE14o- and rat alveolar cells. Eur. J. Pharm. Sci., 6 (Suppl. 1), S95 (Abstract 377), 1998. 157. Needleman, I.G., Martin, G.P., Mather, S.J. and Sobnack, R. Periodontal pocket clearance by gamma scintigraphy. J. Dent. Res., 77, Sp. Iss. B (Abstract 2333), 1998. 158. Hashmi, N., Lansley, A.B., Martin, G.P. and Forbes, B. Mucus secretion reduces testosterone transport across lung and intestinal drug absorption models. AAPSPharmSci., 1 (Suppl.), S-209 (2247), 1998. 159. Zeng, X.M., Martin, G.P. and Marriott, C. The influence of polyvinylpyrrolidone molecular weight on the glass trasition and crystallization of co-lyophilized sucrose. AAPSPharmSci., 1 (Suppl.), S-313 (2607), 1998. 160. Brown, M.B., Hanpanitcharoen, M. and Martin, G.P. The influence of glycosaminoglycans on dermal partitioning of non-steroidal antiinflammatory agents. AAPSPharmSci., 1 (Suppl.), S-383 (3058), 1998. 161. Rao, A., Martin, G.P. and Lansley, A.B. Characterisation of a human bronchial epithelial cell line (16HBE14o-) as a drug absorption model of the airway. AAPSPharmSci., 1 (Suppl.), S-653 (4126), 1998. 162. Rao, A., Forbes, B., Martin, G.P., and Lansley, A.B. Transport of 2 agonists across cultured 16HBE14o-, Caco-2 and rat alveolar cells. J. Pharm. Pharmacol., 51 (Suppl.), 153, 1999. 163. Lim, S.T., Brown, M.B. and Martin, G.P. Evaluation of the mucociliary transport rate of microspheres in vitro. J. Pharm. Pharmacol., 51 (Suppl.), 177, 1999. 164. Amr, S.K, Brown, M.B., Martin, G.P. and Forbes, B. Activity of clindamycin phosphate and clindamycin against organisms of the skin microflora and pilosebaceous isolates. J. Pharm. Pharmacol., 51 (Suppl.), 237, 1999. 165. Tee, S.K., Zeng, X.M., Martin, G.P. and Marriott, C. The role of carrier type and fine particle concentration on the deposition of salbutamol sulphate from dry powder aerosols in vitro. J. Pharm. Pharmacol., 51 (Suppl.), 324, 1999. 166. Zhu, Z.Y., Martin, G.P., Nazir, T., Brown, M.B. The effect of liposome composition and size on the skin partitioning of ciclosporin A. J. Pharm. Pharmacol., 51 (Suppl.), 336, 1999. 167. Forbes, B., Hashmi, N., Martin, G.P. and Lansley, A.B. Effect of drug delivery vehicle on epithelial permeability and mucus secretion. J. Aerosol Med., 12, 138, 1999. 168. Hashmi, N., Matthews, J.L., Martin, G.P., Lansley, A.B. and Forbes, B. Effect of mucus on transepithelial drug delivery. J. Aerosol Med., 12, 139, 1999. 169. Larhrib, H., Martin, G.P., Marriott, C. and Prime, D. Dry Powder inhaler formulations: the influence of engineered lactose crystals upon the deposition of salbutamol. J. Pharm. Pharmacol., 52 (Suppl.), 14, 2000. 170. Liao, Y-H, Brown, M. and Martin, G.P. Biological and chemical assays for the determination of formulated lysozyme activity. J. Pharm. Pharmacol., 52 (Suppl.), 139, 2000. 171. Larhrib, H., Martin, G.P., Marriott, C. and Prime, D. The use of particle engineering to design carriers and drug for respiratory drug delivery. J. Aerosol Med., 14, 120, 2001. 172. Tee, S.K., Martin, G.P., Leeds, A.R., Walker, C., Kicman, A., Cowan, D.A., Fallon, J. and Marriott, C. In vitro/in vivo evaluation of the performance of two lactose carrier-based dry powder aerosol formulations for the delivery of salbutamol to the lung. J. Aerosol Med., 14, 130, 2001. 173. Tee, S.K., Martin, G.P., Leeds, A.R., Walker, C., Kicman, A., Cowan, D.A. and Marriott, C. The effects of fine lactose on the in vitro and in vivo delivery of salbutamol from a dry powder inhaler formulation. Amer. Resp. Crit. Care Med., 163, A163, 2001. 174. Forbes, B., Hashmi, N., Magee, G. and Martin, G.P. In vitro mucus interactions with inhaled drugs. Amer. Resp. Crit. Care Med., 163, A164, 2001. 175. Forbes, B., Larhrib, H. and Martin, G.P. A technique for deliverying respirable powders to respiratory epithelial cells in vitro. Amer. Resp. Crit. Care Med., 163, A165, 2001. 176. Tee, S.K., Marriott, C., Leeds, A.R., Walker, C., Kicman, A., Cowan, D.A. and Martin, G.P. In vivo determination of salbutamol sulphate delivered from a dry powder inhaler formulation in human plasma and urine by solid phase extraction/gas chromatography-mass spectrometry (SPE/GC-MS). British Pharmaceutical Conference Science Proceedings 2001, Pharmaceutical Press (ISBN 0 85369 312 1), London, p.15, 2001. 177. Zaman, M.A., Martin, G.P., Rees, G.D. and Nisbet, M.A. Non-aqueous carbomer formulations for mucosal bioadhesive drug delivery. British Pharmaceutical Conference Science Proceedings 2001, Pharmaceutical Press (ISBN 0 85369 312 1), London, p.66, 2001. 178. Brown, M.B., Lim, S.T., Hanpanitcharoen, M. and Martin, G.P. The effects of hyaluronan and other polymers on the dermal diffusion in vitro of NSAI drugs. British Pharmaceutical Conference Science Proceedings 2001, Pharmaceutical Press (ISBN 0 85369 312 1), London, p.67, 2001. 179. Lim, S.T., Forbes, B., Martin, G.P. and Brown, M.B. Nasal delivery of gentamycin: in vivo evaluation of novel microparticles. British Pharmaceutical Conference Science Proceedings 2001, Pharmaceutical Press (ISBN 0 85369 312 1), London, p.76, 2001. 180. Liao, Y., Brown, M.B. and Martin, G.P. The role of stabilising excipients in preserving the structure of globular proteins during dehydration. British Pharmaceutical Conference Science Proceedings 2001, Pharmaceutical Press (ISBN 0 85369 312 1), London, p.153, 2001. 181. Larhrib, H., Mpanga-Sempa, O., Marriott, C. and Martin, G.P. The effects of micronisation on the particle size and solid state structure of lactose. British Pharmaceutical Conference Science Proceedings 2001, Pharmaceutical Press (ISBN 0 85369 312 1), London, p.253, 2001. 182. Tee, S.K., Martin, G.P., Leeds, A.R., Kicman, A., Cowan, D.A. and Marriott, C. An in vivo evaluation of salbutamol delivery from binary and ternary dry powder inhaler formulations. J. Aerosol Med., 14, 415, 2001. 183. Masoud, O., Marriott, C., Martin, G.P. and Nichols, S. Orophanygeal models as ports of entry for the in vitro assessment of aerosolised drug deposition. J. Aerosol Med., 14, 416, 2001. 184. Liao, Y., Brown, M.B. and Martin, G.P. Protective mechanism of stabilising excipients in the freeze-drying of proteins. AAPSPharmSci., 3 (Suppl.), abstract 769, 2001. 185. Brown, M.B., Lim, S.T. and Martin, G.P. The effect of hyaluronan and other glycosaminoglycans on the biodisposition of diclofenac and ibuprofen when applied topically in vitro. AAPSPharmSci., 3 (Suppl.), abstract 1427, 2001. 186. Nazir, T., Martin, G.P. and Brown, M.B. Dermal delivery of cyclosporin A entrapped liposomal gels - preformulation Franz cell diffusion studies using silastic membranes. AAPSPharmSci., 3 (Suppl.), abstract 1429, 2001. 187. Tee, S.K., Martin, G.P., Leeds, A.R., Walker, C., Kicman, A., Cowan, D.A. and Marriott, C. The effects of fine lactose on the in vivo deposition of salbutamol isomers from a dry powder inhaler formulation. AAPSPharmSci., 3 (Suppl.), abstract 1812, 2001. 188. Lim, S.T., Martin, G.P. and Brown, M.B. A novel method to deliver dry powdered formulations onto cell monolayers. AAPSPharmSci., 3 (Suppl.), abstract 1999, 2001. 189. Royall, P., Martin, G.P., Brown, M., Larhrib, H., Guiziou, B., Andreou, C., Minet, A. and Bakri, A. Characterisation of processing induced changes in morphology by solution calorimetry. Thermometric AB, Application note 2225-02, August 2001. 190. Tee, S.K., Martin, G.P., Leeds, A.R., Walker, C., Kicman, A., Cowan, D.A. and Marriott, C. The influence of a tertiary component on the in vivo disposition of salbutamol isomers aerosolized from a dry powder inhaler formulation. Thorax, 56 (Supplement III), iii61-iii62, 2001. 191. Zaman, M.A., Martin, G.P., Rees, G. D. and Nisbet, M. A. Non-aqueous drug delivery systems: the effect of PEG 400 on bioadhesive function. Proc. Int. Symp. Control. Rel. Bioact. Mater. (Korea), 29, Abstract 448, 2002. 192. Zaman, M.A., Martin, G.P., Rees, G.D. and Royall, P.G. Characterisation of non-aqueous bioadhesive drug delivery systems by differential scanning calorimetry. J. Pharm. Pharmacol., 54 (Suppl.), Abstract 047, S-19, 2002. 193. Liao, Y.H., Brown, M.B., Nazir, T., Quader, A. and Martin, G.P. Investigation of protein denaturation upon spray-drying by differential scanning calorimetry. J. Pharm. Pharmacol., 54 (Suppl.), Abstract 064, S-26-S-27, 2002. 194. Quader, A., Liao, Y.H., Brown, M.B., Martin, G.P., Royall, P. and Forbes, B. Temperature modulated differential scanning calorimetry of a spray-dried model protein:catalase. J. Pharm. Pharmacol., 54 (Suppl.), Abstract 083, S-34-S-35, 2002. 195. Forbes, B., Ghibellini, G., Martin, G.P. and Magee, G.A. CaCo-2 cell monolayer guided optimization of an oral lipid formulation. J. Pharm. Pharmacol., 54 (Suppl.), Abstract 115, S-48-S-49, 2002. 196. Quader, A., Liao, Y.H., Brown, M.B., Martin, G.P., Royall, P. and Forbes, B. Thermal analysis of spray-dried protein formulations. AAPSPharmSci, 4(4), Abstract T2128, 2002. 197. Forbes, B., Ghibellini, G., Martin, G.P. and Magee, G.A. Caco-2 monolayer permeability as an aid to formulation design. AAPSPharmSci, 4(4), Abstract T2174, 2002. 198. Liao, Y.H., Brown, M.B., Nazir, T., Quader, A. and Martin, G.P. The effects of excipients on the stabilization of spray-dried proteins. AAPSPharmSci, 4(4), Abstract W5101, 2002. 199. Yff, B.T.S., Royall, P. G., Martin, G.P. and Brown, M.B. Test materials for solution calorimeters. J. Pharm. Pharmacol., 55 (Suppl.), Abstract 085, S-35, 2003. 200. Akomeah, F. K., Martin, G.P. and Brown, M. B. An in-vitro study of the effect of heat on the percutaneous absorption of three model penetrants. J. Pharm. Pharmacol., 55 (Suppl.), Abstract 087, S-35-S-36, 2003. 201. Akomeah, F. K., Nazir, T., Martin, G.P. and Brown, M. B. The effect of heat on the percutaneous absorption and epidermal retention of methyl paraben, butyl paraben and caffeine: An in vitro study. AAPSPharmSci, 5(4), Abstract T2171, 2003. 202. Jones, S.A., Martin, G.P., Forbes, B. and Brown, M.B. The influence of electrostatic stabilization within apolar suspension formulations. Proc. Int. Symp. Control. Rel. Bioact. Mater., 31, abstract 613, 2004. 203. He, W.J., Brown, M.B. and Martin, G.P. Effect of molecular weight and concentration of hyaluronan on the in vitro release characteristics of diclofenac and ibuprofen across cellulose membrane. J. Pharm. Pharmacol., 56 (Suppl.), abstract 034, S13, 2004. 204. Jones, S.A., Martin, G.P. and Brown, M.B. Stabilisation and delivery of deoxyribonuclease within pressurised hydrofluoralkane metered dose inhaler formulations. The AAPS Journal 6, W5052, 2004. 205. Yff, B.T.S., Brown, M.B., Martin, G.P., Forbes, B. and Royall, P.G. An evaluation of simulated lung fluids using solute partitioning. The AAPS Journal 6, R6056, 2004. 206. Murnane, D., Martin, G.P. and Marriott, C. Investigation of a reverse-phase high performance liquid chromatography (RPHPLC) method for a weakly basic drug. J. Pharm. Pharmacol., 57 (Suppl.), Abstract 180, S-81, 2005. 207. Jones, S.A., Martin, G.P. and Brown, M.B. Manipulation of beclomethasone-hydrofluoroalkane interactions using miscible blends of biocompatible macromolecules. The AAPS Journal Vol. 7, No. S2, Abstract W5074, 2005. 208. Murnane, D., Martin, G.P. and Marriott, C. An HPLC method for concurrent analysis of a bronchodilator and a glucocorticoid. The AAPS Journal Vol. 7, No. S2, Abstract T2020, 2005. 209. Murnane, D., Martin, G.P. and Marriott, C. Control of crystallization of a medicinal product for inhalation therapy using a factorial experimental design approach. The AAPS Journal Vol. 7, No. S2, Abstract W5151, 2005. 210. Zaman, M., Martin, G.P., Nisbet, M. and Rees, G.D. Bioadhesive properties of non-aqueous oral gels. J. Dent. Res. 84 (Spec Iss A):1707, 2005 211. Murnane, D., Martin, G.P., Haley I. and Marriott, C. Evidence for flocculation-controlled microcrystallization of salmeterol xinafoate. J. Pharm. Pharmacol., 58 (Suppl. 1), Abstract 67, A-25-A-26, 2006. 212. Murnane, D., Martin, G.P. and Marriott, C. Particle aggregation of salmeterol xinafoate (SX) in HFA134a high pressure suspensions. J. Pharm. Pharmacol., 58 (Suppl. 1), Abstract 134, A-49-A-50, 2006. 213. Patel, J., Belhadj Salem, L., Martin, G.P., Delattre, L., Evrard, B., Forbes, B. and Bosquillon, C. Use of the MTT assay to evaluate the biocompatibility of β-cyclodextrin derivatives with respiratory epithelial cells. J. Pharm. Pharmacol., 58 (Suppl. 1), Abstract 172, A-64, 2006. 214. Taki, M., Zeng, X.M., Oliver, M., Marriott, C. and Martin, G.P. A comparison of the in vitro deposition profiles of drugs from a combination dry powder inhaler (DPI) using the Next Generation Impactor (NGI). J. Pharm. Pharmacol., 58 (Suppl. 1), Abstract 134, A-65, 2006. 215. Grenha A, Grainger C, Dailey LA, Seijo B, Martin GP, Remuñán-López C, Forbes B. Biocompatibility study of microspheres containing chitosan nanoparticles. Ann. Meeting and Exposition of Con. Rel. Soc. – or Proc. Int. Symp. Control. Rel. Bioact. Mater., 33, abstract 36, 2006. 216. Rees, G.D., Zaman, M.A. and Martin, G.P. Bioadhesion of non-aqueous gels in a dental hard-tissue model. J. Dent. Res. 86 (Spec Iss B): 0157, 2007. 217. Heikal, L., Martin, G.P. and Dailey, L.A. Characterization of the Decomposition Profile of S-Nitrosoglutathione and its Inhibitory Effect on Platelet Aggregation. J. Pharm. Pharmacol., 59 (Suppl. 1), Abstract 27, A-11, 2007. 218. Benaouda, F., Martin, G.P., Jones, S.A. and Brown, M.B. Effect of propylene glycol on in vitro membrane transport of ionised diclofenac diethylamine from saturated binary cosolvent mixtures. J. Pharm. Pharmacol., 60 (Suppl. 1), Abstract 143, A-57, 2008. 219. Heikal, L., Martin, G.P. and Dailey L.A. Novel S-nitrosoglutathione analogues; their physicochemical and pharmacological behavior. Nitric Oxide, 19 (Suppl. 1), 22, 2008. (http://dx.doi.org/10.1016/j.niox.2008.06.011). 220. Zughaid H., Forbes B., Martin G.P. and Patel N. Solubilisation of steroid drugs by simulated intestinal fluids containing lipids. J. Pharm. Pharmacol., 60 (Suppl. 1), Abstract 158, A116-A117, 2009. (http://onlinelibrary.wiley.com/doi/10.1211/002235709789037053/pdf). 221. Benaouda, F., Jones, S., Brown, M. and Martin, G.P. Enhancement of membrane permeation of diclofenac diethylamine using supersaturation. J. Pharm. Pharmacol., 60 (Suppl. 1), Abstract 165, A121-A122, 2009. (http://onlinelibrary.wiley.com/doi/10.1211/002235709789037053/pdf). 222. Zughaid, H., Forbes, B., Martin, G.P. and Patel, N. Effect of simulated intestinal fluid on steroid solubility and permeability across Caco-2 cell monolayers. The AAPS Journal Vol. 11, No. S2, Abstract 863, 2009. (http://www.aapsj.org/abstracts/AM_2009/AAPS2009-000863.PDF) (5) Editorship. Marriott, C. and Martin, G.P. Relevance of mucus to advanced drug delivery. Adv. Drug Del. Rev., 11, 201-403, 1993. (6) Patents. Yianneskis, M., Martin, G.P., Marriott, C., Suen, K.O., Lee, K.C., Ganderton, D. and Timsina, M. Dry Powder Inhalers. US Patent 5,975,076, Nov 2, 1999. International Patent Application Number WO 95/17917, 6 July 1995. European Patent Application 95904631.9 Marriott, C., Martin, G.P. and Brown, M.B. Hyaluronic drug delivery system. United States Patent Application, 27 September 1996. Marriott, C., Martin, G.P. and Zeng, X.M. Improved Crystal Structure. International Patent Publication Number WO 99/48475, 30 September 1999. Larhrib, H., Marriott, C., Martin, G.P., Pritchard, J. and Zeng, X.M. Improved Compositions for inhalation. International Patent Publication Number WO 99/48476, 30 September 1999. Bansal, S.B. and Martin, G.P. Disulphide containing polymers. British Patent application 0100253.4, 5 January, 2001. Zeng, X.M. and Martin, G.P. Pharmaceutical composition for pulmonary delivery. International Patent Publication Number WO 01/58425 A2,16 August 2001. US Appl. No. 10/203,266, Pub. Serial No. US-2003-0152523-A1, 14 Aug 2003 Australian No. 32017/01, European Appl. 01904098.9, PCT Appl. No. PCT/GB01/00489. Jones, S.A., Brown, M.B., Liao, Y.H. and Martin, G.P. Metered dose inhalation preparations. UK Patent Appl. No 02262274.9 Filed 11th Nov 2002 PCT filed 10th November 2003, PCT Appl. No. PCT/GB2003/004836 Publication date 27/05/04 IPN: WO 2004/043442 EPER submitted 10/6/04 UK Patent Jones, S.A., Brown, M.B. and Martin, G.P. Metered dose inhaler preparations of therapeutic drugs UK Patent Application 0328629.1, 11 December 2003. UK Patent Appl. No 0328630.9 Filed 11th December 2003, PCT Appl. No. PCT/GB2004/005172 Filed 10th December 2004 Jones, S.A., Brown, M.B. and Martin, G.P. Metered Dose Inhalation Preparations of peptides and proteins UK Patent Appl. No 0328629.1 Filed 11th December 2003 PCT/GB2004/005206 Filed 10 December 2004. Martin, G.P. and Nokhodchi, A. Recrystallisation of Saccharides. UK Patent Application. 0328537.6, 9 December 2003. Murnane, D. Marriott, C. and Martin, G.P. Micronisation of Inhaled therapeutics. UK Patent Application GB 0511836.9, 2005. Taki, M., Zeng, X.M., Marriott, C. and Martin, G.P. Dry Powder Medicament. UK Patent Application GB 0714134.4, 2008 International Publication Number WO 2009/010770 A2 (Date 22 January 2009). (7) Conference Proceedings. Newbery, R.S., Martin, G.P. and Marriott, C. The effect of certain biliary and duodenal surfactants on mucus structure Proc. 3rd Int. Conference on Mucus and Related Topics (Manchester), p 19, 1988. Batts, A.H., Marriott, C., Martin, G.P. and Bond, S.W. The effect of some preservatives used in nasal preparations on mucociliary clearance Proc. 3rd Int. Conference on Mucus and Related Topics (Manchester), p 21, 1988. Martin, G.P. and Marriott, C. The use of the tracheal pouch to assess the mucoregulatory properties of drugs in vivo Proc. 3rd Int. Conference on Mucus and Related Topics (Manchester), p 21, 1988. El-Hariri, L.M., Martin, G.P. and Newton, R. The effect of pH on the rate of membrane disruption by bile salts. Proc. Int. Symp. Surf. Sol. (Ottawa), 7, P 6.10, 1988. El-Hariri, L.M., Martin, G.P. and Marriott, C. A comparison of the protective effect of some phospholipids in bile salt induced membrane damage. Proc. Int. Symp. Surf. Sol. (Ottawa), 7 , P 6.11, 1988. Hollingsbee, D.A., Martin, G.P. and Marriott, C. The bioavailability of triamcinolone acetonide from a single application of 0.025% cream under a dermatological patch compared to twice daily, unoccluded application of 0.1% cream as assessedby skin blanching. Proc. Ann. Meeting Amer. Acad. Derm. (San Francisco), 48, P166, 1989. Helliwell, M., Martin, G.P. and Marriott, C. Determination of the type and density of functional groups on the surface of microcapsules. Proc. Int. Symp. Microencap. (Glasow), 7, 19, 1990. Helliwell, M., Martin, G.P. and Marriott, C. An in vitro test for the adhesion of microparticulate systems to mucus. Proc. Int. Symp. Microencap. (Glasow), 7, 83, 1990. Marriott, C., El-Hariri, L.M. and Martin, G.P. The toxic effects of biliary surfactants on membranes Proc. 2nd Anglo-Egyptian Conference of Pharm. Sci., p10, 1992 Marriott, C., King, A.J. and Martin, G.P. The possible consequences of mucus turnover in the gastrointestinal tract. Proc. 2nd Anglo-Egyptian Conference of Pharm. Sci., p21, 1992 Shafi, Z.B., Martin, G.P., Olliff, C.J. and James, S.L. Albumin microspheres: tissue distibution after oral administration to rats. Proc. Int. Symp. Microencap. (Dublin), 8, 45, 1992. MacAdam, A.B., Martin, G.P., James, S.L. and Marriott, C. Bovine serum albumin microspheres produced by three techniques: differences in physical appearance and the number of surface groups. Proc. Int. Symp. Microencap. (Dublin), 8, 46, 1992. Malhi, S., Lethem, M.I., Martin, G.P. and James, S.L. The effect of mucus turnover on microparticulate retention in the rat ileum. Proc. 2nd U.S.-Japan Symposium on Drug Delivery Systems (Maui, Hawaii), p31, 1993. Heer, J., Martin, G.P. and Marriott, C. Protective effect of DNA in mucus to gel reduction by bile salts. Proc. 2nd U.S.-Japan Symposium on Drug Delivery Systems (Maui, Hawaii), p45, 1993. Onyechi, J.O., Martin, G.P., Marriott, C. and Murphy, L. Deposition of dry powder aerosols in cascade impactors at different flow rates. Proc. Drug Delivery to the Lungs (London), 4, 28-31, 1993. Haghpanah, M., Marriott, C. and Martin, G.P. Potential use of microencapsulation for sustained drug delivery to the respiratory tract. Proc. Drug Delivery to the Lungs (London), 4, 32-35, 1993. Zeng, X.M., Martin, G.P. and Marriott, C. The delivery of tetrandrine to the lung: optimization of albumin microsphere preparation using factorial design. Proc. 2nd U.S.-Japan Symposium on Drug Delivery Systems (Maui, Hawaii), p58, 1993. Onyechi, J.O., Martin, G.P., Murphy, L., Ganderton, D. and Marriott, C. Calibration of the twin impinger at different flow rates. Proc. 8th Annual Conference of The Aerosol Society (York), p.150, 1994. Sajadi Tabassi, S.A., Martin, G.P. and Marriott, C. The effect of aliphatic mercaptan derivatives on the rheology of mucus glycoprotein gels. Proc. 12th Iranian Congress of Physiology and Pharmacology (Tehran), Int . Relationship Office of Iran University Med. School, p.354, 1995. Brown, M.B., Marriott, C. and Martin, G.P. Hyaluronan: a transdermal drug delivery system. An in vitro investigation. Proc. 7th Int. Seminar on Treatment of Rheumatic Diseases (Tel Aviv), Dec 1995. Brown, M.B., Marriott, C. and Martin, G.P. The effect of hyaluronan on the in vitro deposition of diclofenac within the skin. The 6th Interscience World Conference on inflammation, antirheumatics, analgesia and immunomodulators (Geneva), Abstract 158, 1995. Crooks, J.P., Marriott, C., Martin, G.P., Paget, T.A. and Hassall, D. Colonic adhesion by Entamoeba histolytica: A flow cytometric study. Proc. Soc. Gen. Microbiol., Aberdeen, Sept, 1995. Brown, M.B., Bennett, F.C., Marriott, C. and Martin, G.P. The intradermal localisation of diclofenac using hyaluronan. Proc. 2nd UKCRS Symp. on Controlled Drug Delivery, London, Poster 01, 1996. Crooks, J., Marriott, C. Martin, G.P., Paget, T. and Hassll, D. Monitoring membrane interactions using two colour FACS analysis. Proc. 2nd UKCRS Symp. on Controlled Drug Delivery, London, Poster 03, 1996. Olliver, M.P., Lansley, A.B. and Martin, G.P. The physicochemical characteristics of two gene delivery systems. Proc. 2nd UKCRS Symp. on Controlled Drug Delivery, London, Poster 13, 1996. Olliver, M.P., Lansley, A.B. and Martin, G.P. An investigation of the physical characteristics of two liposomal gene delivery systems. Proc. 1st Int. Symp. on Gene Therapy from a Pharmaceutical Viewpoint, Bath, P 8, 1996. Crooks, J., Marriott,C., Martin, G.P., Paget, T. and Hassall, D. A cytofluorimetric study of entamoeba histolytica plasma memrane adherence to host cell plasma membrane. British Section Soc. Protozoologists (BSSP), London, March, 1996. Needleman, I.G., Martin, G.P., Mather, S.J. and Sobnack, R. Periodontal pocket clearance by gamma scintigraphy. Int. Assoc. of Dental Research, June, 1998. Srichana, T., Brain, A., Martin, G.P. and Marriott, C. The determination of drug-carrier interactions in dry powder inhaler formulations. 5th International Aerosol Conference, Edinburgh, September, 1998. Srichana, T., Martin, G.P. and Marriott, C. Calibration method for the Andersen cascade impactor. 5th International Aerosol Conference, Edinburgh, September, 1998. Rao, A., Forbes, B., Martin, G.P. and Lansley, A.B. Comparison of absorption characteristics of 2 agonists across the airway and alveolar cell culture models. European Respiratory Conference, Geneva, September, 1998. Hashmi, N., Lansley, A.B., Martin, G.P. and Forbes, B. Effect of purinergic stimulation on mucus secretion and Barrier properties of cultured airway epithelial cells. British Association for Lung Research Symposium, Cardiff, September, 1998. Lim, S.T, Brown, M.B. and Martin, G.P. The mucoadhesive potential of novel hyaluronan and chitosan microspheres. Proc. Millenial World Congr. Pharm. Sci., Int. Pharm. Fed., San Francisco, Abstract 22122, 60, 2000. Amr, S.K., Brown, M.B., Martin, G.P. and Forbes, B. The effect of homogenised human skin on the activity of lincosamide antibiotics. Proc. Millenial World Congr. Pharm. Sci., Int. Pharm. Fed., San Francisco, Abstract 22123, 60, 2000. Brown, M.B., Marriott, C., Forbes, B., Hanpanitcharoen, M. and Martin, G.P. The use of hyaluronan in topical drug delivery. Proc. Hyaluronan 2000, International Meeting, Wrexham, UK, Sept, 2000, P 72. Liao, Y-H., Brown, M.B., Nazir, T., Quadir, A. and Martin, G.P. Investigation of lysozyme denaturation upon spray-drying by DSC. Proc. TAC 2002, Chatham, April 2002. Baladda, L., Royall, P., Martin, G.P., Brown, M., Guiziou, B., Duncan, J., and de Velde, G. and Bakri, A. The use of thermal methods to characterize the influence of production parameters on spray dried particles. Proc. TAC 2002, Chatham, April 2002. Quader, A., Liao, Y-H., Brown, M.B., Martin, G.P., Royall, P. and Forbes, B. Thermal analysis of a spray-dried model protein: catalase. Proc. TAC 2002, Abstract 18, Chatham, April 2002. Zaman, M.A., Martin, G.P., Rees, G.D. and Royall, P.G. Thermal analysis of the effect of water on non-aqueous polymer-containing drug delivery systems. Proc. TAC 2002, Abstract 21, Chatham, April 2002. Masoud, O., Martin, G.P., Marriott, C. and Nichols, S. The in vitro assessment of aerosolized drug deposition using novel oropharyngeal models. Proc. Particles 2002, Orlando, Florida, April 2002. Akomeah, F. K., Martin, G.P. and Brown, M. B. Heat and percutaneous absorption: An in vitro study using 3 model penetrants. Skin and formulation, Association de Pharmacie Galenique Industrielle (APGI), 2003, Paris. Nokhodchi, A., Mahsoodi, M., Hassan-Zadeh, D. Barzegar-Jalali, M and Martin, G.P. Mechanical and packing behaviours of recrystallised naproxen from acetone-water solution in presence of various concentrations of hydroxypropylcellulose (HPC) Proc. 2nd Int Conf. Pharmac. Drug Ind. Div, Nat. Res Centre, Abstract PIII-B-37, 233, Cairo, Egypt, 2005. Zaman, M., Martin, G.P., Nisbet, M. and Rees, G.D. Bioadhesive properties of non-aqueous oral gels. IADR, Baltimore J. Dent. Res. 84 (Spec Iss A):1707, 2005 Srichana, R., Seechamnanturakit, V., Kliangklom, A., Srichana, T. and Martin, G.P. A simple molecularly imprinted sorbent assay for ergot binding studies using displacement of unlabeled-(radioligand) dopamine/serotonin. Proc. 4th International Workshop on Molecular Imprinting, Cardiff, 51, 2006. Bodhibukkana, C., Jantarat, C., Tangthong, N., Buaking, K., Srichana, T., Martin, G.P. and Srichana, R. The integration of molecularly imprinted polymer with bacterially derived cellulose membranes for the enantioselective controlled release of racemic propranolol for transdermal delivery. Proc. 4th International Workshop on Molecular Imprinting, Cardiff, 52, 2006. Murnane, D., Martin, G.P. and Marriott, C. Correlation between particle diameter and in vitro deposition from salmeterol xinafoate (SX) high pressure suspensions. APS Inhalation 2007, University of Bath, March 2007. Murnane, D., Martin, G.P. and Marriott, C. Salmeterol xinafoate (SX) microcrystallisation:control by aggregation and flocculation. APS Inhalation 2007, University of Bath, March 2007. Grainger, C.I., Greenwell L.L.,Lockley, D.J., Martin, G.P. and Forbes, B. Presentation of aerosols to cell layers modelling bronchial epithelium. APS Inhalation 2007, University of Bath, March 2007. Taki, M., Zeng, X.M., Marriott, C. and Martin, G.P. A comparison of the in vitro deposition profiles of drugs from a combination dry powder inhaler (DPI) using the multistage liquid impinger (MSLI). APS Inhalation 2007, University of Bath, March 2007. Zughaid, H., Forbes, B., Martin, G.P. and Patel, N. The influence of intestinal fluid composition on drug solubility Proc.7th conference workshop on biological barriers and nanomedicines – advances in drug delivery and predictive technologies (Saarland University; Feb.), 127-128, 2008. Pandolfo, R., Menard, K.P., Martin, G.P. and Royall, P. Application of novel mesh powder pockets for DMA characterisation of crystalline and amorphous lactose powders. Proc. Amorphous Pharmaceutical Materials, The Movenpick Hotel, Amsterdam, September, 2008. Taki, M., Marriott, C., Zeng, X.M. and Marriott, C. The effect of particle size variation on the deposition of drugs from combination dry powder inhalers. APS Inhalation 2009, University of Nottingham, March 2009. Benaouda, F., Jones, S.A., Brown, M.B. and Martin, G.P. Polar water/propylene glycol co-solvent vehicles facilitate optimum ion-pair membrane permeation. Proc. 3rd Symposium on Skin and Formulation and 10th Annual Meeting of the Skin Forum, P-063, 81, Palais des Congrès, Versailles, March 2009. Gan, S.Y., Hider, R.C., Martin, G.P., Jones, S.A., Brown, M.B. and Wade, W.G. Antibacterial properties of zinc-chelator complexes. IADR, Miami, March 2009. (http://iadr.confex.com/iadr/2009miami/webprogram/Session21168.html) Benauoda, F., Jones, S.A., Brown, M.B. and Martin, G.P. Polar water/propylene glycol co-solvent vehicles facilitate optimum ion-pair membrane permeation. Proc. 3rd APGI Symp. Skin Formulation/Skin Forum 10th Ann Meeting, Versailles, March, 2009. Benaouda, F., Jones, S.A., Brown, M.B. and Martin, G.P. Dynamic hydrofluoralkane metered dose aerosols for topical delivery of diclofenac diethylamine, Proc. 2nd PharmSciFair, P-229, 169, Nice, June, 2009. Zughaid, H., Forbes, B., Martin, G.P. and Patel, N. Effect of cholesterol and lipid digestion products on the solubility of two poorly watersoluble drugs. Proc. 2nd PharmSciFair, P-272, 184, Nice, June, 2009. Kaialy, W., Martin, G.P., Ticehurst, M. D. and Nokhodchi, A. Engineered mannitol as potential carrier for enhanced DPI performance. Proc. UK Pharm. Sci. Conference, The Science of Medicines, Nottingham, September 2010. Wade, J.B., Martin, G.P. and Long, D.F. Effects of Moisture on the compaction properties of granular anhydrous tricalcium phosphate (TRI-CAL WG™). Proc. Adv. and Opportunities in Drug Product Manufacturing, Balitimore, MD, September 2010. Benaouda, F., Brown, M. B., Martin, G. P. and Jones, S. A. Manipulation of in situ supersaturation kinetics using superstructured hydrophilic matrices. Proc. UK Pharm. Sci. Conference, The Science of Medicines, Nottingham, August, 2011. S. Jaffari, S., Martin, G. P., Forbes, B., Harding, L. Collins, E. and Murnane, D. Exploring the effect of fine particle interactions on the in vitro deposition of dry powders for inhalation. Proc. UK Pharm. Sci. Conference, The Science of Medicines, Nottingham, August, 2011. Benaouda, F., Martin, G.P., Brown, M.B. and Jones, S.A. The influence of supramolecular structuring of topical delivery vehicles on drug transmembrane penetration. Proc. 13th Int. Conf. Perspectives in Percutaneous Penetration (PPP), La Grande Motte, April, 2012. Martin, G.P. Formulation of Dry Powders for Pulmonary Inhalation: - A combination of Challenges. Proc. Aerosol Soc., Conference, Bristol, April, 2012. Jaffari, S., Collins, E., Murphy, J., Khoo, J., Forbes, B., Martin, G.P. and Murnane, D. Probing Powder De-agglomeration using Dry Dispersion Laser Diffraction. Proc. Drug Delivery Australia, Melbourne, November, 2012.