Malachite Decomposition Lab: Mole Ratios & Mass
advertisement

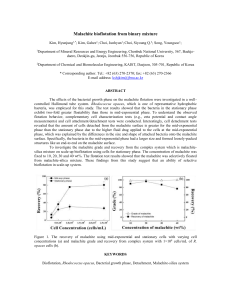
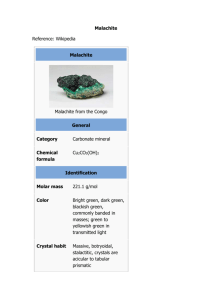
http://educ.queensu.ca/%7Escience/main/concept/chem/c02/C02LAVR2.htm Prior to this activity Review: - Molecular Formulas and Molecular Mass - Amount of Substance in Moles (moles = mass/Molecular Mass) - Chemical Equations ( balancing) MASSES OF REACTANTS AND PRODUCTS HANDOUT~Laboratory : Decomposing Malachite (Toxic)(25 mins.) The IUPAC name for malachite is copper(II) hydroxide carbonate (common name that appears in chemical supply catalogues - basic copper carbonate). Copper(II) hydroxide carbonate is a green double salt with the chemical formula Cu(OH)2 CuCO3(s).It decomposes completely when heated to 200°C according to the following chemical equation: Cu(OH)2 CuCO3(s) _ 2CuO(s) + CO2 + H2O (Before starting the experiment, have students predict what the products may be. After having figured out the products have students write out the balanced equation for the reaction. This is then followed by having students predict how many moles of CuO will be produce if one mole of malachite is used. Ask students to develop a hypothesis) Materials safety glasses, porcelain dish, hot plate, glass stirring rod, malachite (not more than 3g, balance(+0.01g), spatula. Problem How is the mass of copper(II) oxide formed from the decomposition of malachite related to the mass of malachite reacted? (students may work individually or in pairs depending on availability of materials) Experimental procedure 1) Determine the mass of malachite used (assign varying amounts of malachite used, ~ 0.25,0.50,1.25,..,3.00g). 2) Heat sample (stir gently at the same time) until colour changes from green to black. 3) Determine the mass of the black product. Results - record everybody s data in a table form on the blackboard or the overhead. Name moles weight of weight of number of moles number of Malachite used CuO(s) produced of Malachite CuO(s) (g) (g) used produced Data Analysis (discussion in class, 20 mins.) 1) Combine results of each student or each pair of students in a graph (malachite (g) vs. CuO (g)) on a huge piece of square line paper (visible to the class) or on a square line overhead and solve the problem above. Also provide reference data to predict future decomposition. 2) Each student calculate the number of moles of reactant used and also calculate the number of moles of product formed. 3) Compare the mole ratios of malachite to CuO obtained by the students. Questions ~ What is the theoretical mole ratio? Do the results of the students correspond to theoretical value? Explain. How can we predict the number of moles of product produced, given that we know the number of moles of reactants used, in any reaction? Test Students Understanding (10 mins.) ~ From the graph of reference data, predict the mass of CuO produced by decomposing the following masses of malachite used: 0.30g, 1.55g, and 2.70g. ~ Similarly calculate the theoretical values using the mole ratio.(MW of malachite = 221.13g/mol MW of CuO + 79.55g/mol) Solution: malachite _ 2CuO(s) + carbon dioxide + water mole ratio 1 : 2 : 1 : 1 mass 0.30g ? # of moles 0.30 g = 1.36 x 10-3mol m 221.13g/mol m = # of moles of malachite used (mol) x 2 mol 1 mol = 1.36 x 10-3 x 2 mol = 2.72 x 10-3 mol Thus the mass of CuO = 2.72 x 10-3 mol x 79.55 g = 0.22 g 1 mol