UV-Vis Spectrophotometry: Dye Analysis Experiment
advertisement

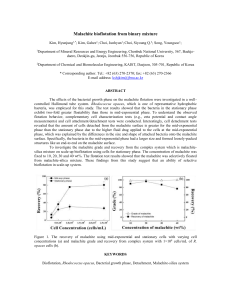
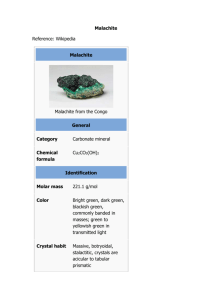
Experiment 1 Title Analysis of dye using UV-Vis spectrophotometer Introduction Synthetic dyestuffs used extensively in textile, printing industries and their effluent being discharged into surface water resource thus led to aquatic ecosystem pollutants. Dye wastewater needs to be treated due to the high chemical and biological oxygen demands, suspended solids and also the colour itself which easily being recognized by the human eye. Adsorption processes are being widely used by various researchers for the removal of dye due to its simplicity, inexpensiveness and efficiency. Chemicals/ Materials 1000 mg/L of malachite green Unknown concentration of malachite green solution Apparatus Procedure Volumetric flask (100 mL, 10 mL) Pipette (1mL, 10mL) Droppers UV-Vis spectrophotometer Quartz cuvettes (UV transparent) Soft tissue (cuvettes wiping) Beaker (100 mL) 1. Prepare 100 mg/L of malachite green solution from stock solution however further dilution need to be conducted in order to have a 10, 8, 6, 4 and 2 mg/L of malachite green for the calibration curve purposes. 2. Record the initial spectra of unknown malachite green using UV-Vis spectrophotometer (618 nm). 3. Determine the concentration of the unknown sample using calibration curve recorded in step 1. Result Concentration of malachite green Colour of malachite green solution Absorbance value 2 mg/L 4 mg/L 6 mg/L 8 mg/L 10 mg/L Unknown concentration Question 1. Draw the calibration curve for the malachite green standard solution. 2. Define ʎmax 3. What is the ʎmax for malachite green solution? 4. What is the concentration of unknown concentration? Reference 1. Liu, Shihao & Wang, Shi & Lei, Chunsheng & Li, Ruyi & Feng, Siyang & Jin, Qiyu. (2022). Study of the efficiency of g-C3N4-loaded P25 for photocatalytic degradation of malachite green in aqueous and Pickering emulsion systems. Journal of Materials Science: Materials in Electronics. 33. 10.1007/s10854022-07767-z.