331 model kit
advertisement
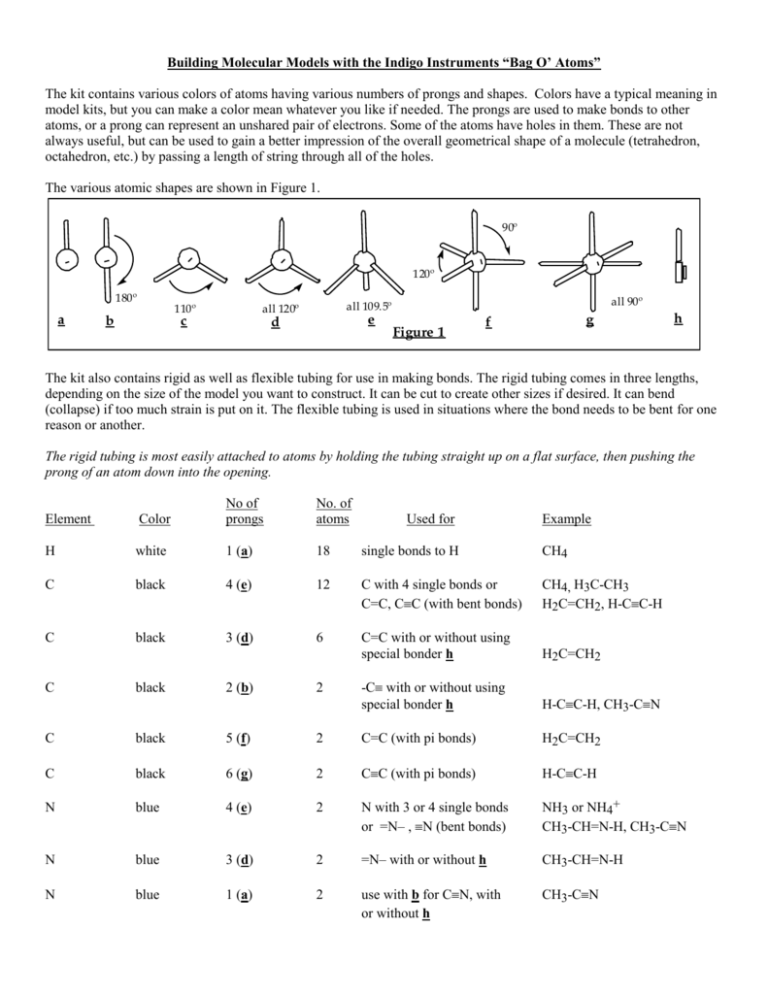
Building Molecular Models with the Indigo Instruments “Bag O’ Atoms” The kit contains various colors of atoms having various numbers of prongs and shapes. Colors have a typical meaning in model kits, but you can make a color mean whatever you like if needed. The prongs are used to make bonds to other atoms, or a prong can represent an unshared pair of electrons. Some of the atoms have holes in them. These are not always useful, but can be used to gain a better impression of the overall geometrical shape of a molecule (tetrahedron, octahedron, etc.) by passing a length of string through all of the holes. The various atomic shapes are shown in Figure 1. 90º 120º 180º a 110º b c all 90º all 109.5º all 120º e d Figure 1 g f h The kit also contains rigid as well as flexible tubing for use in making bonds. The rigid tubing comes in three lengths, depending on the size of the model you want to construct. It can be cut to create other sizes if desired. It can bend (collapse) if too much strain is put on it. The flexible tubing is used in situations where the bond needs to be bent for one reason or another. The rigid tubing is most easily attached to atoms by holding the tubing straight up on a flat surface, then pushing the prong of an atom down into the opening. Element Color No of prongs No. of atoms H white 1 (a) 18 single bonds to H CH4 C black 4 (e) 12 C with 4 single bonds or C=C, CC (with bent bonds) CH4, H3C-CH3 H2C=CH2, H-CC-H C black 3 (d) 6 C=C with or without using special bonder h H2C=CH2 -C with or without using special bonder h H-CC-H, CH3-CN C black 2 (b) 2 Used for Example C black 5 (f) 2 C=C (with pi bonds) H2C=CH2 C black 6 (g) 2 CC (with pi bonds) H-CC-H N blue 4 (e) 2 N with 3 or 4 single bonds or =N– , N (bent bonds) NH3 or NH4+ CH3-CH=N-H, CH3-CN N blue 3 (d) 2 =N– with or without h CH3-CH=N-H N blue 1 (a) 2 use with b for CN, with or without h CH3-CN O red 4 (e) 2 O with 2 or 3 single bonds or =O, O+ (bent bonds) H2O, H3O+ H2C=O, CH3-CO+ O red 2 (c) 4 O with 2 single bonds or =O (bent bonds) H2O, H2C=O (continued) O red 1 (a) 2 S yellow 4 (e) 1 use with b or d for =O, with or without h like oxygen P purple 4 (e) 1 like nitrogen Cl (F, Br, I) green 1 (a) 6 single bonds to halogens special colorless 1 (h) 4 slimline double bonds O=C=O, H2C=O Examples Molecules with all single bonds: These are somewhat obvious and straightforward, and no examples are needed. Note that molecules like H2O and NH3 can be constructed with 4-pronged atoms, with the unused prongs representing unshared electron pairs. Rings with 3 or 4 atoms in the ring itself require the use of the flexible tubing to form bent bonds. Molecules with double or triple bonds: these can be done in more than one way, illustrated below for double bonds, and can be done for any atom that makes double and/or triple bonds. 1. With bent bonds: As shown in Figure 2, two of the prongs of tetrahedral 4-prong atoms are connected with the flexible tubing to give a double bond. A triple bond would have three bent bonds. 2. Using a 3-pronged trigonal atom: As shown in Figure 3, two trigonal atoms are connected with a regular bond as well as with the special bonders (h) to make a slimline double bond. The short, stubby prong of h is pushed into the hole in the trigonal atom, and the other prong is connected to the tubing. The trigonal atoms can be used without the special bonders. The double bond will look like a single bond, but the atom will have the correct geometry. Unfortunately, the drawings of models in textbooks often show double bonds looking like single bonds, which can lead to confusion. A triple bonds in made in a similar fashion using the 2-pronged atoms with 180º prongs (b). 3. Using the 5-pronged or 6-pronged atoms to show a double or triple bond as make up of a sigma bond and one or two pi bonds: As illustrated by the double bond in Figure 4, two 5-pronged atoms are connected with rigid tubing to represent a sigma bond, and two pairs of prongs are connected with flexible tubing the represent the pi bond. A triple bond is constructed in an analogous fashion using 6-pronged atoms. h h e e Figure 2 d d Figure 3 f f Figure 4









