Reactivity of Metals Lab
advertisement

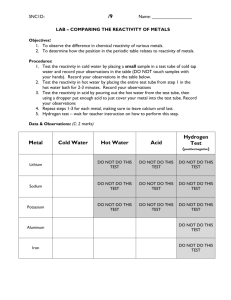
Lab #2 Chapter 11: Reactivity of Metals Lab Name ____________________________ Introduction: The following experiment is designed to demonstrate the relative reactivity of various metals through the combination of solid metals and metal ions in solution. Through observation and recorded findings, the initiation of a single displacement reaction or the lack of reaction will indicate the reactivity of the metals involved. Given the metal activity series, it is possible to predict which combinations will result in a single displacement reaction and which will not react. Relative reactivity observed in this experiment is expected to be consistent with existing metal activity information available. More reactive metals will replace less reactive metals in solution forming a precipitate and metals that show more reactivity than hydrogen will replace hydrogen in an acid to form an ionic solution with the evolution of hydrogen gas. Equipment: 10 medium test tubes 1 test tube rack Reagents and materials: 3 mL Pb(NO3)2 (aq) 3 mL Cu(NO3)2(aq) 3 mL AgNO3 (aq) 3 mL ZnCl2(aq) 3 mL KI(aq) 3 mL NaCl(aq) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 3 mL MgCl2(aq) 3 mL HNO3(aq) Copper wire Magnesium chunks/ribbon Zinc chunks/ribbon Put on lab apron and safety goggles. a. Solutions of lead and copper are toxic b. Silver nitrate is toxic and will leave dark brown stains on skin and clothing Label 10 test tubes # 1-10 and place in the test tube rack. To tube #1 add 3 mL lead (II) nitrate solution and a piece of copper wire. To tube #2 add 3 mL silver nitrate solution and a piece of copper wire. To tube #3 add 3 mL copper (II) nitrate solution and a strip of zinc ribbon To tube #4 add 3 mL lead (II) nitrate and a strip of zinc ribbon. To tube #5 add 3 mL magnesium chloride solution and a strip of zinc ribbon To tube #6 add 3 mL zinc chloride and a chunk of magnesium To tube #7 add 3 mL sodium chloride and a chunk of magnesium To tube #8 add 3 mL potassium iodide and a chunk of magnesium To tube #9 add 3 mL nitric acid and a chunk of magnesium To tube #10 add 3 mL nitric acid and a piece of copper wire. Observe each trial and record observations in table. Record whether or not a reaction occurred based on the formation of a precipitate, evolution or gas, color change of solution etc. Some combinations will not produce a reaction (record as NR). Write complete, balanced chemical equations for each reaction observed. Table 1 Observations of metal activity Tube # Solid metal Metal ion in Solution Observations Write complete, balanced formula equations for each reaction observed. 1._________________________________________________________________________________ 2._________________________________________________________________________________ 3._________________________________________________________________________________ 4._________________________________________________________________________________ 5._________________________________________________________________________________ Analyze data collected and arrange the metals (both solid and in solution) according to their reactivity. List the metals below, from most reactive to least reactive. ___________________________________________________________________________________