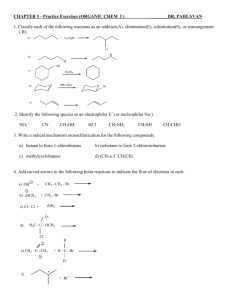
PART II – Show your work

Chemistry 2423
Exam 3A
Hydrocortisone
- Used as an antagonist in the treatment of allergies and
inflammation
1
CHEM 2423 EXAM # 3A (Chapters 8-11)
\
Dr. Pahlavan
PART I- Multiple Choice (3 points each)
____ 1. Compounds contain two or more chiral centers, but are achiral overall, are called:
A. enantiomers compounds B. meso compounds
C. diastereomers D. reference compound
____ 2. How many chiral centers does the following compound have?
CH
3
– CHBr – CHCl – CH
2
– CH
3
A. 1 B. 2 C. 3
____ 3. Consider the following structures, identify the type of isomers.
H CH
3
D. 4
Br H
3
C Br H and
H
3
C H
H
CH
3
Br Br
A. identical B. Constitutional isomers C. diastereoisomers D. enantiomers
____ 4. What is the correct absolute configuration for the following compouds?
CH
3
CN
CH
3
CH
2
A. R B. S
H
C. achiral D. cannot be determined
____ 5. The (+) enanthiomer of a compound has an observed optical rotation of 1.75 degrees
when measure in a one dm tube at a concentration of 0.3 g/ 15 ml. What is thespecific rotation ,
[
], of the molecule?
A. 30
o
B. 45
o
C. 87.5
o
D. 1.75
o
____ 6. Which of the following reagents would be used to complete the following reaction?
CH
3
– CH
2
– CH
2
– C ≡ C - H
CH
3
– CH
2
– CH
2
– CH
2
– CHO
A. 1. BH
3
,THF 2. H
2
O
2
, NaOH , H
2
O B. HgSO
4
, H
2
SO
4
, H
2
O
C. KMnO
4
/ H
3
O
+
D. none of these
2
____ 7. What is the correct configuration for the following compound ?
CH
3
H
Br
Br H
A. (2R,3S)
CH
3
B. (2S,3S) C. (2R,3R) D. (2S,3R)
____ 8. Identify the correct order of the following reaction mechanisms as S
N
1, S
N
2, E1, or E2
I. CH
3
CH
2
Br + H
2
O
CH
3
CH
2
OH + HBr
II. Rate = k[RX] for this elimination reaction
III. Using Nu
–
= Cl
–
or I
–
does not affect the rate of this substitution reaction
IV.
CH
2
Br CH
2
+ KOH + KBr + H
2
O
V. Results in inversion of configuration
A. S
N
2, E2, S
N
2, E1, S
N
1
C. S
N
1, E2, S
N
1, E1, S
N
2
B. S
D. S
____ 9. The transformation is ____________________.
CH
3
N
N
2, E1, S
1, E1, S
N
N
1, E2, S
2, E2, S
CH
3
N
N
2
1
CH
3
- CH - CH
3
CH
3
- C - CH
3
Br
A. an oxidation B. a reduction
C. neither an oxidation nor reduction D. cannot be determined
____ 10. How many allylic hydrogens does the following compound have?
CH - CH
3
A. 3 B. 4 C. 6 D. 7
____ 11. Oxidation of a terminal alkyne with either O
3
or acidic KMnO
4
will yield
__________________________.
A. one mole of a carboxylic acid
C. both A and B
B. one mole of carbon dioxide
D. two moles of carboxylic acids
____ 12. What is the order of increasing oxidation level of the following compounds? (least oxidized to more
oxidized)
I. CH
3
- COOH II. CH
3
CH
3
III. CH
3
- CHO
IV. CH
2
= CH
2
A. I < II < III < IV B. II < IV < III < I C. IV < III < II < I D. III < I < IV < II
3
____ 13. What is the relationship between the following compounds?
OH
O
CH
3
- C = CH - CH
3 and CH
3
- C - CH
2
- CH
3
A. tautomers B. enantiomers
____ 14. Which one of the following is a tertiary alkyl halide?
C. diastereomers D. identical
A. 1-chloro-2-methylpentane
C. 2-chloro-3-methylpentane
B. 2-chloro-2-methylpentane
D. 1-chloropentane
____ 15. Alkyl halides undergo elimination of HX, dehydrohalogenation, on treatment with a _______ .
A. strong base B. weak base C. strong acid D. weak acid
____ 16. A compound that possesses an internal plane of symmetry (where one half of the molecule is the
mirror image of the other half of the molecule) is __________.
A. enantiomeric B. achiral C. optically active D. chiral
____ 17. The organometallic reagent R – Mg – X is called a
A. Gilman reagent B. Simons-Smith reagent C. Grignard reagent D. Ficher reagent
____ 18. How many stereoisomers does the following compound has?
CH
3
OH
Br
Br CH
3
A. 1 B. 2
CH
3
C. 4
____ 19. Which of the following represents vinylic carbocation?
D. 0
CH
2
- CH = CH
2
CH
3
- CH = C - CH
3
CH
3
- CH- C C - H
A. I only
I
B. I and II
II
C. I and III
____ 20. What is the final product of the following reaction?
1) Mg/ether 2) H
2
O
CH 3
- CH
2
- CH
2
Br
A. CH
3
– CH
2
– CH
2
OH B. CH
3
– CH
2
– CH
3
C. CH
3
– CH
2
– CH
2
Mg Br D. CH
3
– CH = CH
2
III
D. II only
4
PART II- Show your work
21. Naming and Structures ( 8 points)
Br
CH
3
- CH
2
- CH
2
- CHBr - C C -H
H
CH
3 CH
2
CH
3
_______________________________ __________________________________
Cis- 1-bromo-3- isopropylcyclohexane 4-chloro-2-hexyne
22. Propose a mechanism to account for the following reaction. Please show the structures of
the intermediates and using curved arrows to indicate electron flow in each step.
(Rearrangement may occur) (2points)
HBr /ether
CH
3
5
23. Complete the following reactions. (20 points)
H
2 a) CH
3
- CH
2
- C C- CH
3
Lindlar catalyst b)
CH
2
- C C - H
HgSO
4
, H
2
SO
4
H
2
O
C C- H c)
1) NaNH
2
2) CH
3
CH
2
Br d) CH
3
- CH
2
- C C - CH
3
KMnO
4
/ H
3
O e) f)
C C- H
2 HCl
CH
2
-CH
2
OH
PBr
3
OH g)
SOCl
2 pyridine
CH
3 h) CH
3
- C = CH - CH
3
Br
Mg /ether H
2
O i) CH
3
- CH
2
- CH - CH
2
Br j)
CH
Br
3
KOH
1) 2 Li, pentane
2) CuI
3) CH
3
-CHBr
CH
3
6
24. Show by a series of reactions how could you prepare the following compounds from the
indicated starting compound. Be sure to clearly indicate the reagents used in each step.
( 10 points)
CH
3
- C C - CH
2
( b)
( a) CH
3
- C C - H
CH - CH
3
CH
3
7
BONUS Question(10 points)- Show all your work. (Do only one of these a or b) a) Draw one enantiomer, and one diastereomer of the following compound.
OH
H
CH
3
H
Br
CH
2
CH
3 b) Given The MS spectrum for a compound with only one oxygen, C x
H y
O , answer the
following questions. a) Determine the position of parent peak and base peak. b) Determine the possible structure of the compound. c) What fragment has the highest intensity?
8
ORGANIC CHEM 2423 EXAM #3A (Answers)
PART I- Multiple Choice (3 points each)
1. B
8. B
2. B
9. A
3. D
10. D
15. A 16.B 17.C
PART II- Show youe work (8 points)
21.
(R) – 2- bromobutane
Br
4. B
11. C
18. B
5. C 6. A
12. B 13.A
19. D 20. B
3 – Bromo – 1- hexyne
7. B
14.B
22.
CH
3
-
3
CH
3
- CH
2
- CH - C C - CH
3
Cl
H - Br hydride shift
Br
23.
CH
3
CH
3 a) c) e)
H
H
CH
3
-CH
2
C = C
C
CH
3 b)
O
CH
2
- C - CH
3
O
C - CH
2
- CH
3 d) CH
3
- CH
2
- C -OH + CH
3-
- COOH
Cl
C - CH
3
CH
2
- CH
2
Br f)
Cl g)
Cl h)
CH
3 i) CH
3
- CH
2
- CH - CH
2
- CH - CH
3 j)
CH
3
CH
3
CH
3
- CH = CH -CH
3
9
CH
3 Br
CH
3
24.
( a) CH
3
- C C - H
NaNH
2
CH
3
- C C -CH
2
CH
2
Br
CH
3
- C C: Na
(b) CH - CH
3
CH
3
Cl
2 light
Cl
2 Li
CuI pentane
Bonus Question (10 points) – Show all your work . a)
Enantiomer
OH
CH
3 H
CH
3
CHBr
CH
3
CH
3
Diastereomer
OH
H
H
Br
H
Br b) parent peak , m/e = 84
CH
2
CH
3 base peak , m/e = 69
CH
2
CH
3
C
5
H
8
O , D.U. = (5) – (8/2) +1 = 2 (two double bond) or one triple bond
Structure:
O
CH
3
- C - CH
2
- CH = CH
2 m/z =84
O
O
C - CH
2
- CH = CH
2 m/z = 69
C
10
- CH
3 m/z =43
Chemistry 2423
Exam 3B
Benzylpenicillin-
is a group of Beta-lactam antibiotics used in the treatment of bacterial infections caused by susceptible, usually Gram-positive, organisms.
11
CHEM 2423 EXAM # 3B (Chapters 8-11)
\
Dr. Pahlavan
DIRECTIONS- Please answer all questions in the space provided as completely and clearly as possible .
Show all your work for the writing portions of the exam.
PART I- Multiple Choice (3 points each)
____1. Which of the following bromoalkanes is secondary alkylhalide?
H CH
3
A. CH
3
CH
2
CH
2
CH
2
Br B. CH
3
CHBrCH
2
CH
3
C. H - C - H D. CH
3
- C - Br
E. CH
3
CH
2
Br
Br
CH
3
____2. Which statement most correctly describes the rate determining step (stable intermediate carbocation) in
the reaction below?
Cl
CH
3
- C = CH - CH
2
- CH
3
+ HCl CH
3
- C - CH
2
- CH
2
- CH
3
CH
3 CH
3
A. Formation of a 3 o carbocation B. Formation of 2 o carbocation
C. Formation of 3 o
radical D. Rearrangement of a 2 o
to 3 o
carbocation
E. Addition of Cl
-
to a carbocation
____3. 1-Butyne (shown below) contains which of the following hybridized orbitals?
H H
H - C C - C - C - H
H H
A. sp
3
only B. sp, sp
2
, and sp
3
C. sp and sp
3
D. sp only E. sp
2
and sp
3
____4. What is the product of the following reaction?
ROOR
CH
3
CH = CH
2
+ HBr
A. CH
3
CH
2
CH
2
Br B. CH
3
- CH - CH
3 peroxide
C. CH
3
- CH - CH
2
Br
D. CH
3
CH
2
CH
3
E. CH
3
- CH - CH
3
Br Br
____5. How many sigma (δ ) and pi (π )bonds are found in ethyne?
A. 2 δ , 2 π B. 3 δ , 2 π
C. 1 δ , 2 π
OOR
D. 4 δ , 1 π
D. 1 δ , 1 π
12
____6. Which of the following best describes the relationship between these two compounds?
CH
3
CH
3 and
CH
3
CH
3
A. Structural isomers B. Enantimers
D. Identical compounds E. Meso compounds
C. Diastereomers
____ 7. According to the Cahn-Ingold-Prelog convention, what is the correct designation of the follown
stereoisomers?
Br
CH
3
C
CH
2
H
CH
3
A. (S) B. ( R) C. (R,S)
____ 8. Which one of the following compounds below is chiral?
O
OH
D. (Z)
CH
3
C - CH
3
C - CH
3
A.
B.
H
C.
CH
3
____ 9. What is the correct IUPAC name of the following compound?
Br
D.
E. Meso
CH
3
OH
E.
O OH
C - C - C
O
HO CH
3
OH
CH
3
- C
C - CH
2
- C - CH
3
CH
3
A. 5-Bromo-2-heptyne B. 3-Bromo-5-heptyne
C. 2-Bromo-2-methyl-4-hexyne D. 5-Bromo-5,5-dimethylhexyne
E. 5-Bromo-5-methyl-2-hexyne
____10. Which of the alkyne addition reactions below involves an enol intermediate?
A. hydroboration/oxidation B. treatment with HgSO
D. hydrohalogenation
4
in dilute H
2
SO
4
C. hydrogenation
E. both A and B
____11. The reagent needed to convert 2-butyne to trans-2-butene is _______________.
A. H
2
/Pt B. H
2
/ Lindlar’s catalyst
C. Li/NH
3
D. Na/NH
3
E. both C and D
13
____ 12. Which of the following compounds is NOT super-imposable on its mirror image?
CH
3
Br H
H H OH
A.
H
3
C CH
3
B.
H Cl
C.
H OH
D.
C
6
H
5
CH
3
E.
H
CH
2
CH
3
Cl
____13. What is the product of the following reaction?
CH
3
Br
OH
Pt
CH
3
- C C - CO
2
H + excess H
2
?
H
OH
H
A.
CH
3
C = C
H
CO
2
H
H CO
2
H
B.
CH
3
E.
CH
3
- C
C = C
H
C - CHO
C.
CH
3
- C C - CH
2
OH
D. CH
3
CH
2
CH
2
CO
2
H
____14. The structure
CO
2
H
H
H
OH
OH
CO
2
H
A. has two chiral and is optically active B. rotates polarized light counterclockwise
C. rotates polarized light clockwise D. has no chiral carbons
E. has a plane of symmetry and is optically inactive
____15. Which of the following represents the intermediate formed in the reaction below?
CH
3
CH
3
H
3
C - C - CH
3
Br
2 uv light
H
3
C - C - CH
3
Br
H
CH
3
CH
3
CH
3
CH
2
CH
3
A.
H
3
C - C - CH
3
B.
H
3
C - C - CH
2
C. H
3
C - C - CH
3
D.
H
3
C - C - CH
3
E.
H
3
C - C - CH
3
H
____16. What is the principle organic product of this reaction sequence?
Br
Mg
H
2
O
CH
3
CH
2
CH
2
Br ether
B. CH
3
CH
2
CH
2
OH C.
CH
3
CH = CH
2
D.
CH
3
CH
2
CHO
A.
CH
3
CH
2
CH
3
14
E.
CH
3
CH
2
COOH
____17. What is the major product of the following reaction?
Cl
2
CH
3
- CH = CH
2
?
CCl
4
A. CH
3
- CHCl - CH
3
B. CH
3
-CHCl - CH
2
Cl C. CH
3
-CH
2
- CH2Cl
____18. Which of the following compound consider to be chiral?
OH
F
OH
A.
B.
C.
H
Br
C Cl
D. CH
2
Cl -CH = CH
2
D. both A and B E. both A and C
E. CH
3
CH
2
CH
3
OH
____19. A newly isolated natural product was shown to be optically active. If a solution of 2.0 g in 10 ml of
ethanol in a 50 cm tube gives a rotation of +2.57
o
, what is the specific rotation of this natural
product?
A. -2.57
o
B. +0.257
o
C. +2.57
o
D. +0.0257
o
E. none of these
____20. A mixtur of equal amounts of two enantiomers are _____________________.
A. is called racemic mixture B. is optically inactive
C. implies that the enantiomers are meso forms D. both A and B D. none of these
PART II – Show your work
16. Naming and structures ( 3 points each) a) Give IUPAC names for the following compounds
CH
3
H
Br
CH
3
- C - C CH
C CH
Cl CH
3
I.
II.
III.
I. _______________________________________ II. _______________________________________
III. ______________________________________ b) Draw structures corresponding to the following systematic names;
(R ) – 2- chlorobutane 2,5,5-trimethyl-3-heptyne
15
17. ( 5 points) Show by a series of reactions how could you prepare the following compounds from the
indicated starting compound. Be sure to clearly indicate the reagents used in each step.
HC CH CH
3
- CH
2
- CH - CH
3
Br
18. ( 5 points) Propose a mechanism to account for the following reaction. Please show the structures of
the intermediates and using curved arrows to indicate electron flow in each step.
(Rearrangement may occur)
HBr /ether
CH
3
19. (5 points) Give an example for the followings;
I. an enol II. a tertiary alkyl halide
III. a terminal alkyne IV. a chiral compound
V. a meso compound
16
20. Predict the major product in each of the following reactions. (2 points each)
CH
2
Br a)
+ CH
3
- O b)
CH
2
CH
3
NBS/CCl
4
OH
PBr
3 c)
HBr/ ether d) f) e)
CH
3
- CH
2
- C C - H
HgSO
4
/H
2
SO
4
H
2
O
C C - CH
3
1. BH
3
, THF
2.H
2
O
2
, OH
Li / NH
3
(liq) g)
C C - CH
3 h) CH
3
- CH
2
C C - CH
3
2 Br
2 i) H C C - CH
3
NaNH
2 j) CH
3
- CHC
CH
3
C - CH
3
KMnO
4
H
3
O
17
CH
2
Br
Bonus Question ( 10 points) – Please show all your work for complete credit. a) Complete the following tree of reactions by giving the major products. Identify structures for compounds
A, B ,C, D, and E.
E
1 - propyne
NaNH
2
A
CH
3
CH
2
Br
B
H
2
/Lindlar's catalyst
1 mole Br
2
/CCl
4
C
KMnO
4
/H
3
O
CH
3
- CHO + compound D
b) Given The MS spectrum for a compound with only one oxygen, C x
H y
O
2
, answer the following
questions. a) Determine the position of parent peak and base peak. b) Determine the possible structure of the compound. c) What fragment has the highest intensity?
18
ORGANIC CHEM 2423 EXAM #3B (Answers)
1. B 2. A 3. C 4. A 5. B 6. D 7. B 8. B 9. E 10. E 11. E
12. D 13.D 14. E 15. A 16. A 17. B 18. C 19. C 20.D
16. a) I. (S) –1-Bromo-1-chloropropane
I.
3,3-dimethyl-1-butyne or t-butyl acetylene2-Ethyl-1-pentene
II.
phenyl acetylene or ethynyl benzene
b)
Cl
CH
3
17.
H
C
CH
3
CH
2
CH
3
CH
3
- CH - C
CH
3
C - C - CH
2
-
CH
3
CH
3
18.
HC
CH
3
CH
2
- C
CH
NaNH
2
CH
3
CH
2
Br
C- H
CH
3
- CH
2
- CH - CH
3
Br
HBr/ether
H
CH
H
2
Lindlar's catalyst
3
CH
2
H
C = C
H
H - Br hydride shift
Br
CH
3
19. Student answers varies
CH
3
OH
I.
CH
3
- C =CH
2
II.
CH
3
- C -Cl
CH
3
CH
3
CH
3
Br
CH
3
III.
CH
3
- C C -H IV.
H
Cl
C OH V.
H
H
20.
CH
2
OCH
3 b)
CH
3 a)
O e) CH
3
- CH
2
- C - CH
3 f)
Br
CH
2
CH
3
OR
O
CH
2
- C - CH
3 or h) CH
3
- CH
2
- CBr
2
- CBr
2
- CH
3 i) CH
3
- C
CH-CH
3
Br c)
Br d)
Br
C - CH
2
-
O
C - CH
2
- CH
3 g)
H
C = C
CH
3
H j) CH
3
- CH - C
O
OH
+ CH
3
C
CH
3
O
OH
19
CH
3
CH
3
OH
OH
Bonus Question
a)
Br CH
2
CH
3
A (CH
3
- C C : Na ) B ( CH
3
- C C - CH
2
- CH
3
) C ( C = C
)
H H
CH
3
Br
O
E.
(
)
D ( CH
3
- C
) C = C
H
CH
3
CH
2
CH
3 b) parent peak , m/e = 84
DU = (4) – (8/2) +1 = 1 db (C=O) base peak , m/e = 43
Fragments appear due to bond cleavage next to C=O (alkyl loss,
.
OCH
2
CH
3
= 45 and CH
3
CO
+
= 43) and
hydrogen rearrangements.
Structure: C
4
H
8
O
2
, MW = 88.11, Ester - Ethyl acetate
O
CH 3
- C - O - CH
2
- CH
3
20