AVERAGE ATOMIC MASS LAB - School District of Clayton
advertisement

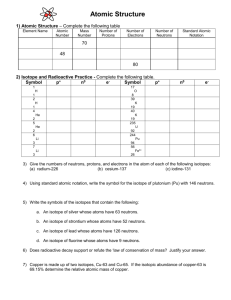
AVERAGE ATOMIC MASS LAB “BEANIUM” Name ____________________________________ Per __________ Date __________ All elements on the Periodic Table exist in at least two isotopic forms. Isotopes are atoms with the same atomic number but with different mass numbers due to varying numbers of neutrons. The atomic mass shown on the Periodic Table for each element, is actually an average of all the isotopes of that element, weighted by the percentage of the abundance in which they occur. NEWS FLASH!!! A NEW ELEMENT HAS BEEN DISCOVERED. Springfield USA—Nuclear Chemists, performing basic research on food products at Springfield Power Plant, have discovered what is believed to be a new element. Mr. Burns, the plant’s owner, says, “We have tentatively named this element Beanium.” Mr. Smithers, assistant to Mr. Burns adds, “We derived this element from the protein nodules we put into our chili.” Further research of the new element will be conducted in more suitable surroundings, namely laboratories in a nearby school. Because Springfield apparently only has an elementary school, research work has been contracted to neighboring Clayton High School. “Student excitement regarding this discovery is running at a fever pitch!” says Lisa Simpson, student. Many chemistry students have generously volunteered their time and expertise to help with the follow-up experiments involving the new element. Dr. Julius Hibbert says the first follow-up experiments conducted at Clayton High School will determine how many isotopes of this element exist. The second experiment will determine the mass of each isotope. The third experiment will determine the percent abundance of each isotope. The final calculations will discover the average atomic mass of the new element. “One unique property of Beanium should make these experiments particularly easy—unlike normal atoms, Beanium atoms are very large.” says Mr. Smithers. “They can be easily seen, and different isotopes can be sorted by hand.” Scientists are expecting a complete, comprehensive summary of this new element within two days, including diagrams and collected data tables. “This is the most exciting Chemistry discovery this century!” exclaimed Mr. Burns. PURPOSE: 1. Identify the number of Beanium isotopes 2. Determine the mass of each isotope 3. Find the percent abundance of each isotope 4. Calculate the average atomic mass of Beanium EQUIPMENT: Balance Sample of Beanium Calculator Procedure: Analyze your individual sample of beanuim in order to complete the following data table. (Also, you are not allowed to consider the mass of the entire sample in solving this problem, as the sample tends to rapidly oxidize, lose electrons, and explode when all isotopes are placed on a balance together.) Data: (Note: You must create your own data table on your paper!!) Total # of beanuim atoms in sample_________ Isotope # of atoms of this isotope present total mass of all the atoms of this isotope Avg. mass of this isotope (show calc.) % abundance of this isotope (show calc.) Whitebeanium Blackbeanium Redbeanium Pintobeanium (has brown spots) Notes: Calculations: Now, using only the last two columns of your data table, calculate the average atomic mass of beanuim. Show your work. Questions: 1. The element magnesium (Mg) has three stable isotopes with the following masses and abundances Isotope Mass (amu) Abundance Magnesium-24 23.985 amu 78.99% Magnesium-25 24.986 amu 10.00% Magnesium-26 25.983 amu 11.01% Calculate the average atomic mass of magnesium from these data. 2. An element is a mixture of two isotopes. One isotope has an atomic mass of 34.969 amu and an abundance of 64.88%. The other isotope has an atomic mass 36.966 amu. Create a data table for this element like the one shown in #1. Calculate the average atomic mass of the element, and then use your periodic table to identify the element. 3. Reflect on all that you have done in this lab activity and briefly explain why the atomic mass reported for each element on the periodic table is not a whole number. 4. Why isn’t the atomic mass of most of the elements on the Periodic Table an integer (not a decimal.) 5. If heaviest isotope was more abundant, and the other two isotopes were less abundant, what would happen to the atomic weight of beanium? Why? Conclusion: Write a conclusion in which you: restate the purpose, summarize your procedure and data recorded, explain the math, and finally give your results. Also, in your conclusion, explain the concept of a weighted average, and how it applies to the determination of the average atomic mass of the elements on the Periodic Table. Remember, here is a great guide for writing your conclusion: 1) Restate the purpose 2) Summarize the procedure and data collection 3) Discuss any important principles / equations used 4) Explain the graphs and/or math 5) Report your results Also, please include any other interesting information you would like to add.