General Medical Officer (GMO) Manual: Clinical Section
advertisement

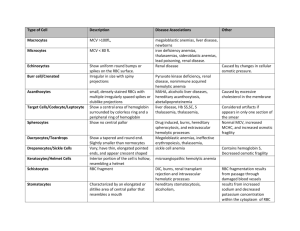
General Medical Officer (GMO) Manual: Clinical Section Anemia Department of the Navy Bureau of Medicine and Surgery Peer Review Status: Internally Peer Reviewed (1) Background Anemia is defined as a decreased level of hemoglobin more than 2 standard deviations below the expected mean for age and sex. In quickly assessing anemia, the presence or absence of symptoms should be determined. Patients with slowly developing anemias will often be completely compensated and have few if any symptoms. Conversely, patients with rapidly developing anemias (i.e., from bleeding, hemolysis) may present with hypotension, tachycardia, weakness, and fatigue. Sometimes the signs and symptoms will point to the diagnosis (pagophagia-ice craving-and iron deficiency; melena-GI bleeding; glossitis and loss of proprioception in B12 deficiency). The family history (sickle cell disease) and exposure history (drugs, toxins, alcohol) are also important. The physical examination may also reveal significant lymphadenopathy, splenomegaly, or heme (+) stools. (2) Initial Evaluation After the patient is determined anemic and the history and physical exam is complete, several simple tests can be performed which may quickly point to the general cause of the anemia. These indicators include the following: (a) Exam the red blood cell indices; these may provide some clues, i.e. microcytosis, macrocytosis. (b) Review the peripheral smear (looking for cell shape i.e., teardrops, fragmented cells, spherocytes, sickle cells, and the presence or absence of abnormal white blood cells). (c) Obtain a reticulocyte count (correct result for hematocrit). (d) Obtain an erythrocyte sedimentation rate (ESR). Most if not all of these tests will be available in field the hospital or on board ship. By carefully following these recommendations, a correct diagnosis can often be determined and an appropriate treatment can be prescribed. (3) Classification of Anemias Most anemias can be classified into three broad categories. (a) Hypoproliferative Anemias These anemias result when the bone marrow is relatively inactive (any condition that causes a decrease in the erythroid activity within the marrow). There are sometimes morphologic clues on the peripheral smear that may indicate these types of anemia (i.e., teardrop cells suggesting marrow replacement with fibrosis, myeloproliferative disorders, cancer, leukemia, and granulomas), nucleated RBCs (also suggesting marrow involvement with cancer, fibrosis, leukemia, granulomas, etc.). Important information to gather from the past medical history include: known history of hypothyroidism or signs and symptoms of the same, a history of heavy ethanol abuse, medication use (sulfas, chloramphenicol, etc.), exposure to radiation, recent viral illnesses (hepatitis), or granulomatous disease (TB). All of these conditions cause a decreased erythroid activity within the marrow. Treatment depends upon the primary cause. (b) Maturation Defects (1) This group results from ineffective production of hemoglobin (cytoplasmic maturation disorders) or ineffective development of nuclear DNA (nuclear maturation disorders). Reviewing the RBC indices often makes diagnosis. (2) Cytoplasmic maturation defect anemias present with microcytosis (usually <70-75 ul). The major causes of anemias in this group are iron deficiency, thalassemia, lead intoxication, B6 deficiency, and inflammatory anemias/anemia of chronic disease (often but not always with a high ESR). (3) There are 2 major causes of nuclear maturation defects: B12 deficiency. Folate deficiency. These patients usually have an MCV > 110 ul. (4) Generally speaking, if your patient has significant anemia, without any symptoms, (suggesting an anemia that has developed very slowly) and has either very small cells or very large cells, the maturation disorders are likely to be a factor. B12, iron, and folate levels can be easily measured. If low, treatment is generally straightforward. (c) Hemolytic Anemias Patients with adequate iron, folate, B12, and no evidence of significant inflammatory disease can respond to the sudden development of anemia with an appropriate production of large amounts of new RBCs to meet acute demands. This rapid production of RBCs is most often reflected as a markedly elevated reticulocyte count. This is a relatively quick way to determine if your patient has suggestive evidence of ongoing hemolysis. When reticulocyte counts are not available, reviewing the blood smear for the presence of an increased number of shift cells (larger pale blue cells) is a good way to estimate the reticulocyte count. If the reticulocyte count is elevated, your patient probably has a hemolytic anemia. Microspherocytes suggest autoimmune hemolysis or hereditary spherocytosis. Schistocytes suggest one of many causes of microangiopathic hemolysis, such as TTP or DIC. If the patient has a hemolytic anemia, consider stopping all non-essential medications, starting an empiric trial of steroids if microspherocytes are present (i.e., prednisone 100 mg/day), and rapid transfer to a tertiary medical treatment facility. (4) Summary In the shipboard or field environment, quickly identify anemias that require rapid transfer to a higher echelon of care, such as those due to blood loss (usually GI bleeding) and due to serious bone marrow disease (i.e., acute leukemia). As always in clinical medicine, a careful history and physical exam plus a review of the blood smear will accomplish this goal the majority of the time without requiring specialized testing. Consultation via telephone, email, or VTC is encouraged if any questions arise. Use the following table as a guide for classifying anemias. Contributing Authors include CAPT George Luiken, MC, USN (1990), and CAPT John C. Phares, MC, USN (1993). Reviewed by CAPT Fred Millard, MC, USN, Department of Hematology/Oncology, Naval Medical Center San Diego, San Diego, CA (1999).