Ch13-RBC - Medical School Pathology
advertisement

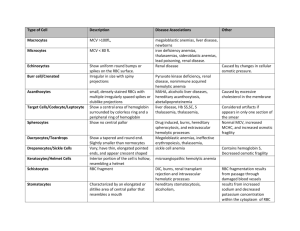
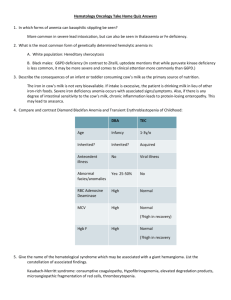
and BLEEDING DISORDERS RBC and Bleeding Disorders • NORMAL – Anatomy, histology – Development – Physiology • ANEMIAS – Blood loss: acute, chronic – Hemolytic – Diminished erythropoesis • POLYCYTHEMIA • BLEEDING DISORDERS * TABLE 13-2 -- Adult Reference Ranges for Red Blood Cells Measurement (units) Hemoglobin (gm/dL) Hematocrit (%) Red cell count (10 /µL) 6 Reticulocyte count (%) Men 13.6–17.2 Women 12.0–15.0 39–49 33–43 4.3–5.9 3.5–5.0 0.5–1.5 Mean cell volume (µm ) MCV 82–96 Mean corpuscular hemoglobin (pg) MCH 27–33 Mean corpuscular hemoglobin concentration (gm/dL) MCHC 33–37 3 RBC distribution width 11.5–14.5 WHERE is MARROW? • Yolk Sac: very early embryo • Liver, Spleen: NEWBORN • BONE – CHILDHOOD: AXIAL SKELETON & APPENDICULAR SKELETON BOTH HAVE RED (active) MARROW – ADULT: AXIAL SKELETON RED MARROW, APPENDICULAR SKELETON YELLOW MARROW MARROW FEATURES • • • • • • • • CELLULARITY 50% MEGAKARYOCYTES at least 1-2/hpf M:E RATIO 3:1 MYELOID MATURATION 1/3 bands or more ERYTHROID MATURATION nucleus/cytoplasm LYMPHS, PLASMA CELLS small percentage STORAGE IRON, i.e., HEMOSIDERIN present “FOREIGN CELLS” MARROW “DIFFERENTIATION” ANEMIAS* • BLOOD LOSS –ACUTE –CHRONIC • IN-creased destruction (HEMOLYTIC) • DE-creased production * A good definition would be a decrease in OXYGEN CARRYING CAPACITY, rather than just a decrease in red blood cells, because you need to have enough blood cells THAT FUNCTION, and not just enough blood cells. Features of ALL anemias • Pallor, where? • Tiredness • Weakness • Dyspnea, why? • Palpitations • Heart Failure (high output), why? Blood Loss Acute: trauma Chronic: lesions of gastrointestinal tract, gynecologic disturbances. The features of chronic blood loss anemia are the same as iron deficiency anemia, and is defined as a situation in which the production cannot keep up with the loss. HEMOLYTIC • HEREDITARY – MEMBRANE disorders: e.g., spherocytosis – ENZYME disorders: e.g., G6PD deficciency – HGB disorders (hemoglobinopathies) • ACQUIRED – MEMBRANE disorders (PNH) – ANTIBODY MEDIATED, transfusion or autoantibodies – MECHANICAL TRAUMA – INFECTIONS – DRUGS, TOXINS – HYPERSPLENISM IMPAIRED PRODUCTION • Disturbance of proliferation and differentiation of stem cells: aplastic anemias, pure RBC aplasia, renal failure • Disturbance of proliferation and maturation of erythroblasts • Defective DNA synthesis: (Megaloblastic) • Defective heme synthesis: (Fe) • Deficient globin synthesis: (Thalassemias) MODIFIERS •MCV, microcytosis, macrocytosis • MCH •MCHC, hypochromic • RDW, anisocytosis HEMOLYTIC ANEMIAS • Life span LESS than 120 days • Marrow hyperplasia (M:E), EPO+ • Increased catabolic products, e.g., bilirubin, serum HGB, hemosiderin, haptoglobin-HGB HEMOLYSIS • INTRA-vascular (vessels) • EXTRA-vascular (spleen) M:E Ratio normally 3:1 HEREDITARY SPHEROCYTOSIS Genetic defects affecting ankyrin, spectrin, usually autosomal dominant Children, adults Anemia, hemolysis, jaundice, splenomegaly, gallstones (what kind?) Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency • A and Mediterranean are most significant types FEATURES of G6PD Defic. • Genetic: Recessive, X-linked • Can be triggered by foods (fava beans), oxidant substances drugs (primaquine, chloroquine), or infections • HGB can precipitate as HEINZ bodies • Acute intravascular hemolysis can occur: – Hemoglobinuria – Hemoglobinemia – Anemia Sickle Cell Disease • • • • Classic hemoglobinopathy Normal HGB is α2 β2: β-chain defects (Val->Glu) Reduced hemoglobin “sickles” in homozygous 8% of American blacks are heterozygous Clinical features of HGB-S disease • • • • Severe anemia Jaundice PAIN (pain CRISIS) Vaso-occlusive disease: EVEREWHERE, but clinically significant bone, spleen (autosplenectomy) • Infections: Pneumococcus, Hem. Influ., Salmonella osteomyelitis THALASSEMIAS • A WIDE VARIETY of diseases involving GLOBIN synthesis, COMPLEX genetics • Alpha or beta chains deficient synthesis involved • Often termed MAJOR or MINOR, depending on severity, silent carriers and “traits” are seen • HEMOLYSIS is uniformly a feature, and microcytic anemia, i.e, LOW MCV (just like iron deficiency anemia has a low MCV) • A “crew cut” skull x-ray appearance may be seen in severe erythroid hyperplasia. Hemoglobin H Disease • Deletion of THREE alpha chain genes • HGB-H is primarilly Asian H HIGH • HGB- has a affinity for oxygen • HGB-H is unstable and therefore has classical hemolytic behavior HYDROPS FETALIS • FOUR alpha chain genes are deleted, so this is the MOST SEVERE form of thalassemia • Many/most never make it to term • Children born will have a SEVERE hemolytic anemia as in the erythroblastosis fetalis of Rh disease: – Pallor (as in all anemias), jaundice, kernicterus – Edema (hence the name “hydrops”) – Massive hepatosplenomegaly (hemolysis) Paroxysmal Nocturnal Hemoglobinuria (PNH) GlycosylphosPhatidylInositol (lipid rafts) • ACQUIRED, NOT INHERITED like all the previous hemolytic anemias were • ACQUIRED mutations in phosphatidylinositol glycan A (PIGA) • Note: It is “P” and “N” only 25% of the time! Immunohemolytic Anemia • All of these have the presence of antibodies and/or compliment present on RBC surfaces • NOT all are AUTOimmune, some are caused by drugs • Antibodies can be – WARM (IgG) – COLD AGGLUTININ (IgM) – COLD HEMOLYSIN (paroxysmal) (IgG) IMMUNOHEMOLYTIC ANEMIAS • WARM AGGLUTININS (IgG), will NOT agglutinate at room temp – Primary Idiopathic (most common) – Secondary (Tumors, especially leuk/lymph, drugs) • COLD AGGLUTININS: (IgM), WILL agglutinate at room temp – Mycoplasma pneumoniae, HIV, mononucleosis • COLD HEMOLYSINS: (IgG) Cold Paroxysmal Hemoglobinuria, hemo-LYSIS in body, ALSO often follows mycoplasma pneumoniae COOMBS TEST • DIRECT: Patient’s CELLS are tested for surface Ab’s • INDIRECT: Patient’s SERUM is tested for Ab’s. HEMOLYSIS/HEMOLYTIC ANEMIAS DUE TO RBC TRAUMA • Mechanical heart valves breaking RBC’s • MICROANGIOPATHIES: –TTP –Hemolytic Uremic Syndrome NON-Hemolytic Anemias: i.e., DE-creased Production • • • • • • • • “Megaloblastic” Anemias B12 Deficiency (Pernicious Anemia) Folate Deficiency Iron Deficiency Anemia of Chronic Disease Aplastic Anemia “Pure” Red Cell Aplasia OTHER forms of Marrow Failure MEGALOBLASTIC ANEMIAS • Differentiating megaloblasts (marrow) from macrocytes (peripheral smear, MCV>94) • Impaired DNA synthesis • For all practical purposes, also called the anemias of B12 and FOLATE deficiency • Often VERY hyperplastic/hypercellular marrow Decreased intake Inadequate diet, vegetarianism Impaired absorption Intrinsic factor deficiency Pernicious anemia Gastrectomy Malabsorption states Diffuse intestinal disease, e.g., lymphoma, systemic sclerosis Ileal resection, ileitis Competitive parasitic uptake Fish tapeworm infestation Bacterial overgrowth in blind loops and diverticula of bowel Increased requirement Pregnancy, hyperthyroidism, disseminated cancer Vit-B12 Physiology • Oral ingestion • Combines with INTRINSIC FACTOR in the gastric mucosa • Absorbed in the terminal ileum • DEFECTS at ANY of these sites can produce a MEGALOBLASTIC anemia Please remember that ALL megaloblastic anemias are also MACROCYTIC (MCV>94 or MCV~100), and that not only are the RBC’s BIG and hyperplastic/hypercellular, but so are the neutrophils, and neutrophilic precursors in the bone marrow too, and even more so, HYPERSEGMENTED!!! PERNICIOUS ANEMIA • • • • • • • • MEGALOBLASTIC anemia LEUKOPENIA and HYPERSEGS JAUNDICE NEUROLOGIC posterolateral spinal tracts ACHLORHYDRIA Can’t absorb B12 LOW serum B12 Flunk Schilling test, i.e., can’t absorb B12, using a radioactive tracer FOLATE DEFICIENCY MEGALOBLASTIC AMEMIAS • • • • • • Decreased Intake: diet, etoh-ism, infancy Impaired Absorption: intestinal disease DRUGS: anticonvulsants, BCPs, CHEMO Increased Loss: Hemodialysis Increased Requirement: Pregnancy, infancy Impaired Usage Fe Deficiency Anemia • Due to increased loss or decreased ingestion, almost always, in USA, nowadays, increased loss is the reason • Microcytic (low MCV), Hypochromic (low MCHC) • THE ONLY WAY WE CAN LOSE IRON IS BY LOSING BLOOD, because FE is recycled! Fe Transferrin Ferritin (GREAT test) Hemosiderin Clinical Fe-Defic-Anemia • Adult men: GI Blood Loss • PRE menopausal women: menorrhagia • POST menopausal women: GI Blood Loss 2 BEST lab tests: • Serum Ferritin • Prussian blue hemosiderin stain of marrow (also called an “iron” stain) Anemia of Chronic Disease* • CHRONIC INFECTIONS • CHRONIC IMMUNE DISORDERS • NEOPLASMS • LIVER, KIDNEY failure * Please remember these patients may very very much look like iron deficiency anemia, BUT, they have ABUNDANT STAINABLE HEMOSIDERIN in the marrow! APLASTIC ANEMIAS • ALMOST ALWAYS involve platelet and WBC suppression as well • Some are idiopathic, but MOST are related to drugs, radiation • FANCONI’s ANEMIA is the only one that is inherited, and NOT acquired • Act at STEM CELL level, except for “pure” red cell aplasia APLASTIC ANEMIAS APLASTIC ANEMIAS • • • • • CHLORAMPHENICOL OTHER ANTIBIOTICS CHEMO INSECTICIDES VIRUSES – EBV – HEPATITIS – VZ MYELOPHTHISIC ANEMIAS • Are anemias caused by metastatic tumor cells replacing the bone marrow extensively POLYCYTHEMIA • Relative (e.g., hemoconcentration) • Absolute – POLYCYTHEMIA VERA (Primary) (LOW EPO) – POLYCYTHEMIA • • • • (Secondary) (HIGH EPO) HIGH ALTITUDE EPO TUMORS EPO “Doping” CVAC, the trendy California bubble pods P. VERA • A “myeloproliferative” disease • ALL cell lines are increased, not just RBCs BLEEDING DISORDERS (aka, Hemorrhagic “DIATHESES”) • Blood vessel wall abnormalities √ • Reduced platelets √ • Decreased platelet function √ • Abnormal clotting factors √ • DIC (Disseminated INTRA-vascular Coagulation), also has ↓ plats. VESSEL WALL ABNORMALITIES (angiopathic thrombocytopenias) (NON-thrombotic cytopenic purpuras) • • • • • Infections, especially, meningococcemia, and rickettsia Drug reactions causing a leukocytoclastic vasculitis Scurvy, Ehlers-Danlos, Cushing syndrome Henoch-Schönlein purpura (mesangial IgA deposits too) Hereditary hemorrhagic telangiectasia (Osler–Weber– Rendu syndrome, Autosomal Dominant) • Amyloid THROMBOCYTOPENIAS • Like RBCs: –DE-creased production –IN-creased destruction –Sequestration (Hypersplenism) –Dilutional • Normal value 150K-300K DE-CREASED PRODUCTION • • • • • • APLASTIC ANEMIA ACUTE LEUKEMIAS ALCOHOL, THIAZIDES, CHEMO MEASLES, HIV MEGALOBLASTIC ANEMIAS MYELODYSPLASTIC SYNDROMES (PRELeukemias) IN-CREASED DESTRUCTION • • • • • • • AUTOIMMUNE (ITP) POST-TRANSFUSION (NEONATAL) QUINIDINE, HEPARIN, SULFA MONO, HIV DIC, “CONSUMPTIVE” TTP/HUS “MICROANGIOPATHIC” THROMBOCYTOPENIAS • ITP (Idiopathic Thrombocytopenic Purpura) • Acute Immune • DRUG-induced • HIV associated • TTP, Hemolytic Uremic Syndrome • • • • I.T.P. ADULTS AND ELDERLY ACUTE OR CHRONIC AUTO-IMMUNE ANTI-PLATELET ANTIBODIES PRESENT • INCREASED MARROW MEGAKARYOCYTES • Rx: STEROIDS ACUTE ITP • • • • CHILDREN Follows a VIRAL illness (~ 2 weeks) ALSO have anti-platelet antibodies Platelets usually return to normal in a few months DRUGS • Quinine • Quinidine • Sulfonamide antibiotics •HEPARIN HIV • BOTH DE-creased production AND IN-creased destruction factors are present Thrombotic Microangiopathies • BOTH are very SERIOUS CONDITIONS with a HIGH mortality: – TTP (THROMBOTIC THROMBOCYTOPENIC PURPURA) – H.U.S. (HEMOLYTIC UREMIC SYNDROME) • These can also be called “consumptive” coagulopathies, just like a DIC “QUALITATIVE” platelet disorders • Mostly congenital (genetic): – Bernard-Soulier syndrome (Glycoprotein-1b deficiency) – Glanzmann’s thrombasthenia (Glyc.-IIB/IIIA deficiency) – Storage pool disorders, i.e., platelets misfunction AFTER they degranulate • ACQUIRED: ASPIRIN, ASPIRIN, ASPIRIN BLEEDING DISORDERS due to CLOTTING FACTOR DEFICIENCIES • NOT spontaneous, but following surgery or trauma • ALL factor deficiencies are possible • Factor VIII and IX both are the classic X-linked recessive hemophilias, A and B, respectively • ACQUIRED disorders often due to Vitamin-K deficiencies (II, VII, IX, X) • von Willebrand disease the most common, 1% von Willebrand Disease • • • • 1% prevalence, most common bleeding disorder Spontaneous and wound bleeding Usually autosomal dominant Gazillions of variants, genetics even more complex • Prolonged BLEEDING TIME, NL platelet count • vWF is von Willebrand Factor, which complexes with Factor VIII, it is the von Willebrand Factor which is defective in von Willebrand disease • Usually BOTH platelet and FactorVIII-vWF disorders are present PTT PT/INR • • • • • • HEMOPHILIA A The “classic” HEMOPHILIA Factor VIII decreased Co-factor of Factor IX to activate Factor X Sex-linked recessive Hemorrhage usually NOT spontaneous Wide variety of severities • Prolonged PTT (intrinsic) only • Rx: Recombinant Factor VIII • • • • • HEMOPHILIA B The “Christmas” HEMOPHILIA Factor IX decreased Sex-linked recessive Hemorrhage usually NOT spontaneous Wide variety of severities • Prolonged PTT (intrinsic) only • Rx: Recombinant Factor IX DIC, Disseminated INTRA-vascular, Coagulation • • • • ENDOTHELIAL INJURY WIDESPREAD FIBRIN DEPOSITION HIGH MORTALITY ALL MAJOR ORGANS COMMONLY INVOLVED DIC, Disseminated INTRA-vascular, Coagulation • Extremely SERIOUS condition • NOT a disease in itself but secondary to many conditions – Obstetric: MAJOR OB complications, toxemia, sepsis, abruption – Infections: Gm-, meningococcemia, RMSF, fungi, Malaria – Many neoplasms, acute promyelocytic leukemia – Massive tissue injury: trauma, burns, surgery • “Consumptive” coagulopathy Common Coagulation TESTS • PTT (intrinsic) • PT INR (extrinsic) • Platelet count, aggregation • Bleeding Time, so EASY to do • Fibrinogen • Factor Assays RBC LAB http://www.chronolab.com/hematology/2_1.htm