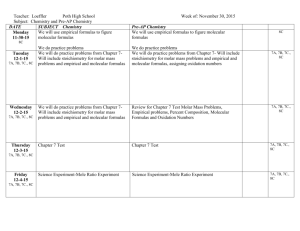
Empirical/Molecular Formulas
advertisement

Empirical and Molecular Formulas p.16 Complex!!! 1) The molecular formulas of some substances are as follows. Write their empirical formulas a) Acetylene, C2H2 (used in oxyacetylene torches). (CH) b) Glucose, C6H12O6 (the chief sugar in blood). (CH2O) c) Octane, C8H18 (a component of gasoline). (C4H9) d) Ammonium nitrate, NH4NO3 (a fertilizer component).(NH4NO3) 2) A radioactive form of sodium pertechnetate is used as a brain-scanning agent in medical diagnosis. An analysis of a 0.9872 g sample found 0.1220 g of sodium and 0.5255 g of technetium. The remainder was oxygen. Calculate the empirical formula of sodium pertechnetate. (Use the value of 98.907 as the atomic mass of Tc and arrange the atomic symbols in the formula in the order NaTcO.) sample xg(Na) xg(Tc) xg(O) (i) Mass(O) = 0.9872 –(0.1220 + 0.5255) = 0.3397 g Element Mass(g) Mole Ratio Na 0.1220 23.0 = 0.005304348 Tc 0.5255 98 = 0.005362245 1.010 1 O 0.3397 16.0 = 0.02123125 4.002 4 1 Empirical Formula NaTcO4 3) Potassium persulfate (Anthion®) is used in photography to remove the last traces of typo from photographic papers and plates. A 0.8162 g sample was found to contain 0.2361 g of potassium and 0.1936 g of sulfur; the rest was oxygen. The molecular mass of this compound was measured to be 270. What are the empirical and molecular formulas of potassium persulfate? (Arrange the atomic symbols in the formulas in the order KSO.) sample xg(K) xg(S) xg(O) Mass(O) =0.8162 –(0.2361 + 0.1936)=0.3865 Element Mass(g) Mole Ratio Empirical Formula K 0.2361 39.1= 0.006038363 S 0.1936 32.1= 0.006031153 1.001=1 KSO4 1 0.3865 16.0 = 0.02415625 4.005=4 MF = (EF) x MM = (EF) x 270 = (KSO4) x 270 = (39.1 + 32.1 + 64.0) x 270 = 135.2 x x = 1.997 = 2 Molecular Formula: (KSO4) X 2 = K2S2O8 4) Adenosine triphosphate (ATP) is an important substance in all living cells. A sample with a mass of 0.8138 g was analyzed and found to contain O 0.1927 g of carbon, 0.02590 g of hydrogen, 0.1124 g of nitrogen, and 0.1491 g of phosphorus. The remainder was oxygen. Its molecular mass was determined to be 507. Calculate the empirical and molecular formulas of adenosine triphosphate. (Arrange the atomic symbols in alphabetical order in the formulas.) sample xg(C) xg(H) xg(N) xg(P) xg(O) Mass(O) = 0.8138 –(0.1927 + 0.02590 + 0.1124 + 0.1491)= 0.3337 Element Mass(g) Mole Ratio Empirical Formula C 0.1927 12.0 = 0.016058333 3.338 X 3 = 10 H 0.02590 1.0 = 0.02590 5.3849 X 3 = 16 N 0.1124 14.0 = 0.008028571 1.669 X 3 = 5 C10H16N5P3O13 P 0.1491 31.0 = 0.004809677 1 X3 = 3 O 0.3337 16.0 =0.02085625 4.336 X 3 = 13 MM = (EF) x 507 =(C10H16N5P3O13) x 507 = [10(12.0) + 16(1.0) + 5(14.0) + 3(31.0) + 13(16.0)]x 507 = 507x x=1 EF = MF = Molecular Formula: C10H16N5P3O13 5) Realgar (re-AL-gar) is a deep red pigment used in painting. A 0.6817 g sample was found to contain 0.4774 g of arsenic; the remainder was sulfur. The molecular mass of realgar was found to be 428. What are the empirical and molecular formulas of this pigment? (Arrange the symbols in the order AsS.) sample xg(As) xg(S) Mass(S) = 0.6817 –0.4774= 0.2043 Element Mass(g) Mole Ratio Empirical Formula As 0.4774 74.9 = 0.006373832 S 0.2043 32.1= 0.006364486 MF = (EF) x MM = (AsS) x 428 = (74.9 + 32.1) x 428 = (107) x x=4 Molecular Formula: (AsS) X 4 = As4S4 1.001=1 AsS 1 6) Isobutylene is a raw material for making synthetic rubber. A sample with a mass of 0.6481 g was found to contain 0.5555 g of carbon; the rest was hydrogen. Its formula mass was determined to be 57. What are the empirical and molecular formulas of isobutylene? (Place the atomic symbols in the formulas in the order CH.) sample xg(C) xg(H) Mass(H): 0.6481 -0.5555= 0.0926 Element Mass(g) Mole C 0.5555 12.0= 0.046291667 H 0.0926 1 = 0.0926 MF = (EF) x Ratio Empirical Formula 1 CH2 2 MM = (CH2) x 57 = (CH2) x 57 = (12 + 2) x 57 = 14 x x=4 Molecular Formula: (CH2) X 4 = C4H8 = isobutylene 7) Cyanuric acid is used for such different purposes as making synthetic sponges and killing weeds. A sample with a mass of 0.5627 g was found to contain 0.1570 g of carbon, 0.01317 g of hydrogen, and 0.1832 g of nitrogen, with the balance being oxygen. Its molecular mass was found to be 129. Calculate the empirical and molecular formulas of cyanuric acid, arranging the atomic symbols in alphabetical order. Sample xg(C) xg(H) xg(N) xg(O) Mass(O) = 0.5627 –(0.1570 + 0.01317 + 0.1832) =0.20933 g Element Mass(g) Mole Ratio Empirical Formula C 0.1570 12.0 = 0.01308333 1.00001 CHNO H 0.01317 1 = 0.01317 1.00664 N 0.1832 14.0 = 0.013085714 1.0002 O 0.20933 16.0 = 0.013083125 1 MF = (EF) x MM = (EF) x 129 = (CHNO) x 129 = (1 + 12 + 14 + 16) x 129 = 43 x x=3 Molecular Formula: (CHNO) X 3 = C3H3N3O3 8) Hypophosphoric acid is one of several acids containing both oxygen and phosphorus. Its formula mass is 162. A sample with a mass of 0.8821 g was found to contain 0.0220 g of hydrogen and 0.3374 g of phosphorus, with the remainder being oxygen. Calculate its empirical and molecular formulas. (Arrange the atomic symbols in the formulas in the order HPO.) Mass(O) = 0.8821 – (0.0220 + 0.3374) = 0.5227 Element Mass(g) Mole H 0.0220 1 = 0.0220 P 0.3374 31.0 = 0.010883871 O 0.5227 16 = 0.03266875 MM = (EF) x 162 = (H2PO3) x 162 = (2(1.0) + 31.0 + 3(16.0)) x Ratio Empirical Formula 2.02 H2PO3 1 3 162 = (81) x x=2 Molecular Formula: (H2PO3) X 2 = H4P2O6 Answer to Questions p.17 1a)CH b)CH2O c)C4H9 d)NH4NO3 2)NaTcO4 3)KSO4, K 2 S 2 O8 4)C10H16N 5P3O13 5)AsS, As4S4 6)empirical:CH2 molecular: C4H8 7)empirical: CHNO molecular: C3H3N3O3 8)empirical: H2PO3 molecular: H4P2O6