MOLECULAR GEOMETRY: POLAR MOLECULES
advertisement

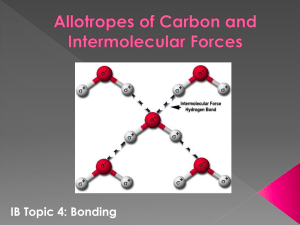
MOLECULAR GEOMETRY: POLAR MOLECULES The molecular geometry of a molecule will determine whether a molecule is polar or non-polar. The presence of a polar bond within the molecule is not enough to determine the overall polarity of the molecule; the shape also affects the outcome as well. Example: Carbon dioxide The bonds of carbon dioxide are polar yet the overall molecule is non-polar O=C=O The magnitudes of the polar bonds are the same and effectively cancel each other. Example: Consider the following PCl5, SF6, CCl4 The geometric orientations of the molecules in the above examples provide a zero net dipole moment. Summary: A molecule that contains polar bonds will not necessarily be a polar molecule. If the shape of a molecule is such that the polar bonds are symmetrically charged, their effects many cancel and the molecule will be considered non-polar. Symmetrical molecules are non-polar. Symmetrical molecules include: Linear, Trigonal planar, tetrahedral, trigonal bipyramidal, octahedral. Molecular crystals Properties of molecular crystals 1. Neither liquid nor solid conduct electricity. 2. Many exist as a gas at room temperature and atmosphere. a. Of those that are solid or liquid are relatively volatile. 3. Melting point of solid crystals are relatively low. 4. Boiling point of liquids are relatively low. 5. Solids are generally soft and have a waxy consistency. 6. Large amount of energy is required to chemically decompose a substance into simpler substances. Notes: Point #1 refers to both polar and non-polar molecules o Polar molecular crystal: molecules on the lattice points are polar o Non-polar molecular crystal: molecules on the lattice points are non-polar Points #2-5 refer to intermolecular forces Points #6 refers to intramolecular forces Intramolecular Forces Force WITHIN an individual molecule Intermolecular Forces Forces BETWEEN molecules Types of Intermolecular Forces There are 3 types 1. Dipole –Dipole Forces i. Found in a molecule with a charge separation (a dipole) ii. Molecules line up in crystal such that opposite dipole are next to each other. iii. Result is molecules are held together and an increased amount of energy is required to separate molecules (i.e. higher b.p) iv. Strength of attraction depends on the size of the charges on the molecules. 2. Van der Waal Forces (a.k.a London Forces…a.k.a dispersion forces) i. A temporarily induced dipole as a result of the molecule moving. ii. As molecules moves, electron density shifts (like water in a balloon) creating a dipole. iii. At that point small dipole-dipole interaction occurs. iv. Depends on the number of electrons and size of the molecules. v. Weaker than dipole-dipole interaction. vi. Molecules that only experience vdw forces have lower boiling point. 3. Hydrogen Bonding i. ii. iii. iv. v. Do not involve sharing of electrons (not covalent) Do no bind to ions together (not ionic) Too electrostatic in nature to be vdw forces Is a special case of dipole-dipole interaction but much stronger More like the interaction between two molecules in which they share a proton (unequally) vi. To have hydrogen bonding you MUST have a lone pair of electrons. Volatile: ability of a liquid to vapourize (in most volatile liquids, the molecules have enough energy to escape the liquid phase and enter the gaseous phase, for example, gas)