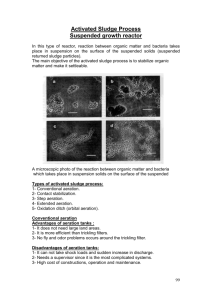
Table 10-5 gives general design parameters
advertisement

53:157 Environmental Engineering Design Biological Processes for the Removal of CBOD5, TAN, and NO3Reading: “Fundamentals” of biological processes - Ch. 8 of M&E and Sections 12.1 thru 12.9 of V&H Design for removal of CBOD - Ch. 10 of M&E, sections 12.12 thru 12.30 in V&H, and Ch. 90 in GLUMRB design standards Design for removal of TAN and NO3- Sections 11-5 thru 11-9 of M&E and 14.11 thru 14.14 of V&H General Process Description: Food + Bacteria + Nutrients End-Products + More Bacteria In Aerobic processes, O2 is required as a terminal electron acceptor. CBOD and NH4+ are typically removed using Aerobic processes: CaHbOcNd + O2 CO2 + H2O + NH3 + bacteria NH4+ + 2O2 NO3- + 2H+ + H2O + bacteria If “complete” nitrogen removal is needed, NO3- can be removed by biological denitrification, which is an anaerobic (sometimes called anoxic) process. However, an electron donor (energy source) must be available for the process to work. With domestic wastewater, it is typically the wastewater organics that serve as the electron donor: CaHbOcNd + NO3- N2 + CO2 + H2O + OH- + bacteria 1 For biological processes to work, certain requirements must be met. These were summarized as the “check list of requirements for bacterial growth” in 53:155: 1. Carbon source (waste to be treated?) 2. Electron donor (energy source – waste to be treated?) 3. Electron acceptor - O2 (aerobic) - NO3- (anaerobic (anoxic)) - SO42- (anaerobic (sulfate reducing)) - CO2 (anaerobic (methanogenic)) 4. Macronutrients - N (C5H7O2N – bacteria are about 12% N) - P (bacteria are about 1-2% P) - for aerobic processes: BOD/N/P 100/5/1 - for anaerobic processes: somewhat less depending on electron acceptor 5. Micronutrients - trace metals like Fe, Zn, Cu, Mo, etc. - other trace components (e.g., S, vitamins, etc.) 6. “Proper” pH 7. “Proper” temperature 8. Absence/Control of toxic substances 9. Adequate contact 10. Adequate time We will initially focus on aerobic processes for CBOD and TAN removal. You must remember that biological treatment is a conversion process; you are in general converting soluble (and “non-settleable”) molecules into solids (bacteria) which then must be removed (almost always by sedimentation) to ensure removal efficiency. There are in general two types of processes: (1) suspended growth and (2) attached growth. With suspended growth, mixing is provided to keep the bacteria and waste in suspension and well mixed. With attached growth, a surface (plastic, rocks, etc.) is provided for bacterial attachment and 2 entrapment. An additional difference is in the practice and function of recycle. With suspended-growth systems, we are recycling bacteria (solids) to control the Mean Cell Residence Time (MCRT; sometimes called Solids Retention Time (SRT or c)) and separate it from the Hydraulic Retention Time (HRT or ). With attached-growth systems we are typically recycling treated wastewater to control the hydraulic loading rate and dilute the incoming waste to insure that oxygen does not limit removal. Schematically, the differences are shown below: Suspended Growth schematic: Aeration Tank O2 Treated Effluent Settling Tank Recycle Waste Sludge Attached Growth schematic: Biological Reactor O2 Treated Effluent Settling Tank Recycle Waste Sludge 3 So, the four main components of aerobic biological processes designed for the removal of CBOD and TAN are: 1. 2. 3. 4. Biological reactor (waste enters here & O2 must be supplied) Oxygen delivery system (“natural” vs “engineered”) Sedimentation basin Recycle pumps Again, we must recall that biological treatment is a CONVERSION process, as demonstrated by this simplified schematic and mass balance: 4 ACTIVATED SLUDGE 1. A suspended-growth process, with O2 as the electron acceptor, which can be used to remove both CBOD and NOD (Nitrogenous Oxygen Demand). In fact, with proper design, it can be a combined aerobic/anoxic process that can also remove NO3--N and P. 2. O2 is provided using either air or pure oxygen (which is typically generated on-site). The gas-transfer system is either by mechanical aeration or by diffused aeration. 3. Settled sludge is recycled from the settling tank to the aeration tank to maintain a desired c (MCRT) and MLSS (MLVSS). This operation also separates “solids” retention time (MCRT) from “liquid” retention time (HRT). 4. There are many activated-sludge process modifications to choose from. Basic information is contained in: - M&E Table 10-3 gives a brief, general description - M&E Table 10-4 givers general operating characteristics - Table 10-5 gives general design parameters It should be noted that many of the process modifications have a plug-flow regime and differ only in how and where the O2 is provided and how and where the influent waste is added (i.e., “step-feed”). We will focus primarily on the “complete-mix” and “plug-flow” hydraulic regimes. We will spend a bit of time discussing SBRs. In an attempt to give you information that will be at least somewhat useful in working the Problem Sets, I’ll spend a bit of time on three of the main components of suspended-growth aerobic processes: (1) supplying adequate O2, (2) sizing the aeration basin, and (3) coordinating the settler and aeration basin via sludge recycle. 5 Design of the O2 supply system: Must maintain a minimum DO in the aeration basin to insure proper process operation (e.g., insure good settling, maintain aerobic conditions, etc.). Must provide sufficient electron acceptor (O2) for growth of the requisite bacteria. This requirement is determined by “process stoichiometry”: CaHbOcNd + O2 CO2 + H2O + NH3 + bacteria In order to design the aeration system, the oxygen requirements must be determined (lbs O2/day). In M&E, the following equation is used for this purpose (Eq. 10-5): MOR = Q(S0 S)(8.34) lbs O 2 = - 1.42PX f day S0 = mg/L of BOD5 entering the aeration basin, S = mg/L of BOD5 leaving the aeration basin, Q = flow entering the aeration basin in MGD, f = conversion factor for converting BOD5 to BODL PX = waste activated sludge production in lbs VSS/day lb/MG Recall – 8.34 mg/L PX can be estimated using several equations, for example Eqs. 10-3 and 8-44 of M&E: PX = YobsQ(S0-S)(8.34) Eq. 10-3 Y 1 k d c Eq. 8-44 Yobs = 6 Yobs = “observed” bacterial yield in lb VSS/lb BOD5 Y = “true” bacterial yield in lb VSS/lb BOD5 kd = bacterial decay coefficient in day-1 c = solids retention time (MCRT) in days Values for Y and kd can be found in M&E Table 8-7 for “typical” domestic wastewater. Once MOR has been determined, the oxygen-supply system (aeration system) can be designed. We will focus on mechanical aeration and diffused aeration using air. You should be “aware” that pure O2 can be used, and often is. In this case, the pure O2 is generated on site and typically introduced into a covered aeration tank. Mixing is typically provided using mechanical devices. Please refer to pp. 574-578 for details. For the material given below, I’m assuming that fundamentals of gas transfer have been covered in other courses (53:151, 53:155, 53:156). For review, see M&E pp. 276-287. General schematics of the two types of aeration systems will be shown in class via overheads. Mechanical Aeration Design of mechanical aeration systems typically involves converting manufacturer’s information developed under standard aeration test conditions (1 atm, 20o C, clean water, C0 = 0 mg/L) to field conditions. Mechanical aerators are typically “low-speed” or “hi-speed”. Details are given in M&E on pp. 569-574. The following equation is typically used: C walt C ) (Eq. 10-19) Cs N = O2 transferred under field conditions (lbs O2/Hp-hr), N = N0(1.024)T-20()( 7 N0 = O2 transferred under standard test conditions (lbs O2/Hp-hr), T = field temperature (o C), = oxygen-transfer correction factor (see M&E Table 10-10), = salinity-surface tension correction factor, Cwalt = saturation DO concentration (mg/L) for clean water at field T and altitude (see M&E App. E and Fig. 10-17), C = operating DO concentration (mg/L), Cs = saturation DO concentration (mg/L) for clean water under standard test conditions (9.17 mg/L). Once a value for N is calculated, and the Hp for the mechanical aerator is known (usually given by manufacturer), the number of aerators can be calculated. Example: Surface aerator with N0 = 4.0 and nameplate Hp = 50 MOR = 6,500 lb/day C = 2 mg/L (why??) T = 28o C Elevation = 5,000 ft = 0.95 = 0.85 How many aerators are required?? 8 Diffused Aeration Design of a diffused aeration system involves selecting the type of diffuser (e.g., coarse vs fine-bubble usually), and making calculations similar to those for mechanical aeration. That is, information is usually available from equipment manufacturers (or Table 10-7 in M&E) for oxygen transfer under standard test conditions. The procedure then involves conversion to field conditions and calculating the number of diffusers required and the blower capacity need to deliver the required air. We will focus on fine-bubble diffused aeration systems because they are usually the most efficient and economical. With fine-bubble diffusers, the air supply typically has to be cleaned (filtered) to help prevent clogging the diffusers. Details are given in M&E on pp. 556569. Also see Example 10-2. Perhaps best to learn from an example?? Let’s say that a ceramic-dome diffuser gives the following performance data: SOTE = 28% @ 2.0 scfm per diffuser Require delivery of 6,500 lbs O2/day (like mech. aeration example) How many diffusers are needed and what scfm needs to be delivered? 9 Now, how would one covert this to field conditions and determine the blower Hp required? The SOTE value of 28% needs to be converted to field conditions and the above numbers re-calculated. After this is done, one needs to re-calculate the scfm required and use this value in equations like the ones on p. 565 of M&E. More than one blower will likely be needed. Oxygen Required for Nitrification How does the approach above change when you are trying to accomplish removal of TAN (nitrification)? The answer is that only the lbs O2/day changes (i.e., MOR increases to include the mass of oxygen required to accomplish nitrification). Recall the overall stoichiometry: NH4+ + 2O2 NO3- + 2H+ + 2H2O This reaction tells us that 4.57 lbs of O2 are required for every lb of TAN oxidized (you should know how to get this number!). Now, NOR = 4.57(Q)(N0-N)(8.34) – 1.42 PXN N0 = influent TAN (mg/L as N), should TKN be used? N = effluent TAN (mg/L as N), PXN = lbs/day of VSS from nitrifying bacteria In some texts, the “1.42” is replaced by “1.98”, assuming the N in C5H7O2N (bacteria) is oxidized all the way to NO3-. The use of 1.98 is more “correct” while the use of 1.42 is more conservative. Determination of PXN is done in a manner analogous to that for PX using values of Y and kd that are appropriate for nitrifying bacteria (Table 1116; also see Example 11.1) For separate-stage nitrification, the above equation (NOR) would be used to calculate the O2 requirements, which in turn would be used to size the aeration equipment. 10 For single-stage CBOD-NOD removal, the O2 required is NOR + MOR, the sum of which gives M&E equation 10.6: lbs O2/day = Q(S0 S)(8.34) + 4.57(Q)(N0-N)(8.34) – 1.42 PX f Using this number, the mechanical or diffused aeration system can be designed. Sizing the Aeration Basin There are a variety of data, equations, and assumptions required for sizing the aeration basin. There are some good examples in M&E of these process calculations (e.g., examples 10-2, 10-3, and 11-1). We will do some brief calculations in class. Additional detail will be required for Problem Set 7. We will first do what I will call “gross” calculations that are pretty good F first-cuts at design calculations. They begin with the definition of , M the “Food-to-Microorganism” ratio: QS0 S F = = 0 VX M X Q = flow to aeration basin (not including recycle!), S0 = BOD to aeration (mg/L), V = volume of aeration basin, X = MLSS or MLVSS (mg/L), V = nominal hydraulic retention time (HRT) = . Q 11 F lb BOD lb BOD are or . M lb MLVSS - day lb MLSS - day The BOD can be either BOD5 or BODL. So, be sure to be consistent with units when using this parameter. A useful “factoid” to keep in mind is that MLVSS is typically 0.7 to 0.85 times MLSS. M&E uses 0.8. The most common units for Design using this parameter typically involves selecting (assuming) a F value for - typical values are 0.2 to 0.5, although values from 0.1 to M 0.7 are sometimes seen (even lower than 0.1 for nitrification). Then one selects X (or ) and calculates (or X). Of course, Q and S0 are “known”. The usual practice is to select an X. For example, let’s say that Q = 5 MGD and S0 = 100 mg/L BOD5. What aeration tank volume is required to treat this wastewater to our Design Problem’s treatment goal? We’ll work this problem in class. 12 Design of the Final Clarifier Part of the “job” of the final clarifier is to provide sludge of sufficient concentration to maintain the desired MLVSS (“selected” above as part of the aeration tank design). The equation used is an approximation of a mass balance around the aeration tank: X R= where Xr X X = MLVSS or MLSS Xr = VSS or SS of the recycle flow recycle flow rate Q R = recycle ratio = r = (M&E use for R) influent flow rate Q The term Xr is also called Xw, which is the solids concentration in the waste activated sludge (WAS). It is “obvious” that Xr = Xw. Well designed, well-operating secondary clarifiers can produce Xr values ranging from 8,000 to 15,000 mg/L. Simple design calculations involve selection of Xr (or better yet, a range of Xr values) and the calculation of R from the above equation. Recycle pumps can then be sized from these calculations. To size the clarifiers, one need to “select” a design overflow rate, calculate the area, and then check the solids loading rate to see if it is acceptable. Alternatively, a solids loading rate can be assumed, an area calculated, and then the overflow rate (hydraulic loading rate) check. Finally, a design depth is selected and the hydraulic retention time checked. Table 10-12 of M&E gives typical values for overflow rates, solids loading rates, and depths. We will do a simple example in class demonstrating these calculations. X (mg/L) 3,000 3,000 2,000 2,000 Xr (mg/L) 8,000 15,000 8,000 15,000 R 0.60 0.25 0.333 0.154 Qr (MGD) 3.00 1.25 1.667 0.769 13 Note the effect of changes in X and Xr on R. Perhaps a good idea to provide excess recycle pumping capacity to handle changing settling characteristics. Now, what about sizing the secondary clarifiers? Table 10-12 contains information that will be useful. Please note that when calculating overflow rate, Q is used in conjunction with clarifier area, while when calculating solids loading rate, Q+Qr is used. Now, select type (rectangular vs circular), size (diameter or length & width), select depth and check HRT, design weirs and check WLR, and design solids collection mechanisms. Please note that the fundamentals of sedimentation analyses are covered on pp. 229-240 of M&E, and the “solids flux approach” to design is demonstrated in Example 10-2. These details should have been covered in 53:155 and 53:156. 14 A more “Fundamental” Approach to Design For an excellent example of this approach, refer to M&E Example 10-2. I will briefly review these concepts below. For simplicity, I will use a “complete-mix” system to demonstrate the approach. All approaches come from combining a reactor type with a kinetic expression and then constructing a mass balance (53:151, 53:155, 53:156). Several “new” equations are needed which relate BOD removal (S) and/or TAN oxidation (N) to growth of bacteria (X). The so-called “Monod approach” is used. K s (1 k d c ) (see Table 8-7 for typical values of k, Ks, Y, kd) c (Yk - k d ) - 1 Y(S0 - S) X = ( c )( ) 1 k d c VX c = (“e” represents effluent; “w” represents waste) Qe X e Q w X w X X R=( )(1 ) (recall that Xr = Xw) Xr - X Xr - X c S= The procedure here is roughly the same. These are steps that are typically taken: Select c (typical values in Table 10-5) Calculate S using the first equation above Select X, then calculate and then V from the second equation Select an Xw (= Xr) to achieve in the secondary clarifer Calculate the WAS rate (Qw) to do this from the third equation (you will need to assume a value of Xe (typically 10-30 mg/L) 6. Now calculate R 7. Calculate MOR 1. 2. 3. 4. 5. 15 From this analysis, you can determine the size of the aeration tanks, the size of the sedimentation tanks, and the size of the O2-supply equipment. In addition, you have determined the flow of WAS to be treated by your solids management system (QwXw). Please note that you need to know both the VSS and SS of the WAS to design the solids management system. For details on single- and separate-stage nitrification, see Section 11-6 and Example 11-1 in M&E. Aerobic Attached-Growth Processes There are two general options here: (1) trickling filters (M&E Section 10-5) and (2) rotating biological contactors (M&E Section 10-6). There are many varieties. For example, M&E lists the following trickling filter options: low-rate filters intermediate-rate filters high-rate filters super high-rate filters roughing filters Table 10-13 gives typical design data for all listed types of filters. The two general types of media are rocks and plastic. Rock trickling filters are typically 6-10 feet in depth while plastic trickling filters (sometimes called “biotowers”) can be 10-40 feet deep (lighter material). Typical media are shown in Fig. 10-33. In addition, there are combined suspended growth-attached growth processes. These are discussed in M&E Section 10-7. Be sure to at least look over this section and become familiar with the terminology. These are aerobic processes where O2 serves as the electron acceptor. O2 is supplied “naturally” in most cases. Our focus will be on trickling 16 filters, although you are responsible for understanding the general operating principles of the other attached growth and combined processes. The air-flow through a trickling filter occurs due to temperature differences between the air and the wastewater (pp. 622-624). The waste is generally applied using rotating applicators (Fig. 10-32; one rotating arm or two rotating arms). The rotational speed of the arms and the dosing rate are important variables (see pp. 617-619). Biofilters can be designed using the typical loading data in Table 10-13 or with empirical equations developed through experience. Sometimes these design equations are given by equipment manufacturers; other times consultants develop them from pilot-scale studies. The following is an example of such an empirical equation (watch units): ln Se D = -k20 Si (Q V ) n Se = total BOD5 in settled effluent from the filter (mg/L), Si = total BOD5 in wastewater applied to the filter (mg/L), k20 = waste treatability “rate” constant @ 20o C ((gal/min)n-ft D = depth of the filter (ft) Qv = hydraulic loading rate to filter (gpm/ft2 = Q/A) 17 There are many of these types of equations (53:151 and 53:155 may have used different ones). One must be careful in using them – you need to understand whether recycle is included in Qv (or whatever symbol is used for hydraulic loading rate) and whether Si is the BOD5 (or BODL) of the wastewater coming from the primary settler or Si is diluted with S RSe effluent from the secondary settler (in this case, Si = 0 ). 1 R There is a good example (Ex. 10-7) in M&E using this approach. There is an error on p. 626 under 1 (d). They have switched Se and Si. It should read as follows: Se = 30 mg/L and Si = 550 mg/L. This example may help you in working Problem Set 7. Another way to make basic design calculations is using the design data in Table 10-13. This is acceptable for homework design problems providing you justify your choices. Example: Compare the area requirements for (1) a low-rate trickling filter that will ensure nitrification and (2) a high-rate tricking filter that will give approximately 85% BOD5 removal without nitrification. What depths would you recommend? Assume the flow is 2.5 MGD, the BOD5 is 175 mg/L, and TAN = 22 mg/L. 18 Sequencing Batch Reactors (a brief description) Fill-and-draw activated sludge system (aeration and clarification carried out sequentially in same tank) Five-step cycle (typically lasting 4 to 6 hours): 19 ADVANTAGES* (More flexibility): Serves as equalizing basin during fill mode (can tolerate greater peak flows or shock loads of high BOD). Can achieve sequential nitrification (aerobic) and denitrification (anaerobic), in the same tank. Periodic discharge of effluent allows holding it until specified requirements are met Mixed liquor is not washed out by hydraulic surges Better settling of sludge (quiescent, easier to control filamentous growth by F/M and DO adjustment) DISADVANTAGES* Requires sophisticated timing units and sensors (which can freeze in winter) and larger aeration equipment Greater demand for operator attention Clogging of air diffusers by settled sludge Need oversized effluent sewers (high intermittent decant flow) Not very feasible for Q > 10 MGD *Relative to conventional activated sludge 20 21