Answers to practice for Midterm Five(MSWord document)
advertisement

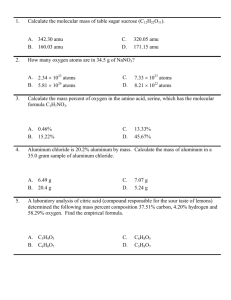
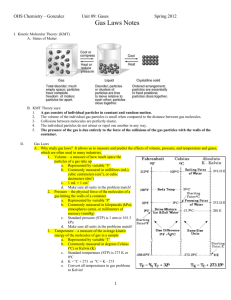
Practice for Midterm 5 Chem 1000 2004 Your responsible for the following sections of the text: Chapters 5.1-5.7 Representative questions: 5.59, 61, 63, 66, 68, 70, 73. R = 0.08205 L•atm/mol•K 1 J = 1 kg•m2 sec-2 No = 6.022 x 1023 mol-1 , , me = 9.11 x 10-31 kg R = 8.314 J/mol•K Question One A mixture of H2 (g) and O2 (g) is prepared by electrolyzing 1.32 g of water, and the mixture of gases is collected over water at 30°C when the barometric pressure is 748 mmHg. The volume of "wet" gas obtained is 2.90 L. What must be the vapor pressure of water at 30°C? 2 H2 O (l) O2 ( g) + 2 H2 (g) 1.32 g H2O will produce 0.11 mol of H2 and O2. This will result in a pressure of 717 mmHg. The difference between this and 748 mmHg is the partial pressure of water: 31 mmHg. Question Two A sample of liquid nitrogen trichloride was heated in a 1.50 liter closed container until it decomposed completely to gaseous elements. The resulting mixture exerted a pressure of 744 torr at 75¯C. a) What is the partial pressure of each gas in the container? b) What was the mass of the original sample? 2 NCl3(g) –––> N2 (g) + 3 Cl2 (g) P-N2 is 186 mmHg or 0.245 atm P-Cl3 = 558 mmHg or 0.734 atm Mass = 3.09 g Question Three A sample of iodine (I2) vapour effuses at a rate of 15.0 mg/h. Under the same conditions, uranium fluoride is found to effuse at a rate of 17.7 mg/h. What is the molecular formula of uranium fluoride? Show your work. Caution: rates must be used in units of moles per time, not mass per time. RateI MI RateUF 2 2 n MUF MI n 2 M UF n The fractions on the left convert the effusion rates into moles/h. You need only solve for MUFn. N = 6. Question Two The Haber Process is the principal method for fixing nitrogen (converting N2 into nitrogen compounds). N2 ( g) + 3 H2 (g) 2 NH3 (g) Assume complete conversion of the reactant gases to NH3 (g) and that the gases behave ideally. i.) What volume of NH3 (g) can be produced from 313 L of H2 (g) if the gases are measured at 515°C and 525 atm pressure? V NH3 = 209 L ii.) What volume of NH3 (g), measured at 25°C and 727 mmHg, can be produced from 313 L H2 (g), measured at 515°C and 525 atm pressure? 4.34 x 104 L Question Eight A mixture of H2 and Ne is placed in a 5.00 L flask at 20ºC. The partial pressure of the H2 is 1.4 atm and the partial pressure of the Ne is 2.1 atm. What is the mole fraction of H2? 0.40 Question One (four marks) A mixture of 21 moles of SO2 and 15 moles of O2 gas reacts to form SO3 gas. i) If the initial total pressure is 8.4 atm, what is the partial pressure of O2 before any reaction occurs? PO2 = 15/(15+21)x8.4atm = 3.5 atm. ii) At the point at which the no. of moles of SO3 is 6.0 moles, what is the mole fraction of O2? 2 SO2 + O2 —> 2 SO3 If the quantity of SO3 is 6 moles, then 6 moles of SO2 must have been consumed leaving 15 moles SO2. At the same time 3 moles of O2 would have been consumed leaving 12 moles. The mole fraction of O2 is therefore = 12/(12+6+15) = 0.36 Question Two (four marks) i) Solid sulfur has the molecular formula S8. In the gas phase, however, sulfur has a different structure. What is the formula for this form of sulfur given that it effuses 1.51 times more slowly than nitrogen gas, N2. Show your work. The molar atomic mass of S is 32.1 g/mol. Rate N2/Rate Sx = 1.51 = (MSx/MN2)1/2 24.2 g/mol x (1.51)2 = MSx = 64 g/mol. The formula must be S2. ii) Physicists have cooled Rubidium atoms to a temperature very near absolute zero to create what is known as a Bose-Einstein condensate. What is the rms speed of Rb atoms (M=85.5 g/mol) at this temperature (0.00024 K)? urms = (3RT/M)1/2 with M converted to kg/mol. =(3x8.31J/molx 0.00024K/85.47g/molx10-3kg/g)1/2 = 0.26 m/S