(catechol oxidases) assay - Science and Plants for Schools
advertisement

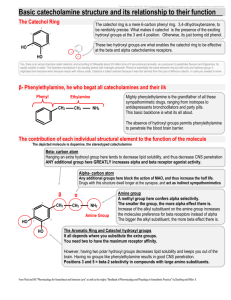
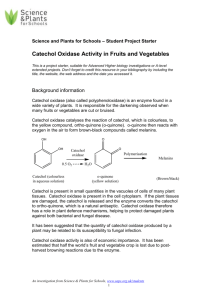
Polyphenoloxidase (catechol oxidases) assay Technical & Teaching Notes Browning of the cut surface of some fruits and vegetables is due the presence of a group of enzymes called polyphenoloxidases. These enzymes are released by the broken cells and they catalyse the reaction between colourless molecules called polyphenols and molecular oxygen. This reaction creates coloured compounds and these new compounds can spontaneously cross react with one another to form black-brown complexes called melanins. One example of a substrate for these enzymes is catechol, hence the alternative name ‘catechol oxidases’ for these enzymes. Catechol is oxidised initially to the orange compound benzoquinone which is then converted to melanins. The conversion to melanin is spontaneous but slow. Polyphenoloxidase (slowly) catechol + oxygen --------------------> benzoquinone + water --------------------> melanins Food processing and cooking often involve procedures which are intended to inhibit the action of polyphenoloxidases. Why do you think a cook immediately places freshly peeled potatoes into a pan of water? Or why do people squeeze a few drops of lemon juice on to a freshly cut avocado? Mushrooms contain high levels of polyphenoloxidases, so how do you think pre-sliced packaged ones can be prevented from going brown? The assay In this technique, the change from a colourless solution of catechol to coloured benzoquinone is followed with a colorimeter. A fruit extract is added to a solution of catechol and the rate of formation of coloured benzoquinone is measured. The faster the rate of increase in absorbance of the reaction mixture, the greater the polyphenoloxidase activity of the fruit extract. 1. Make an extract of fruit or vegetable, by grinding in a mortar or blending with an equivalent mass of water. 2. Strain the extract through muslin, then centrifuge the filtrate to remove the remaining solids. (Alternatively, the filtrate could be filtered using a Buchner funnel, or just allowed to stand - so the solids form a sediment at the bottom of the vessel. Then draw the liquid off into another vessel.) Note that if the plant tissues contain much polyphenoloxidase activity, then the extract itself will become quite darkly coloured — but this need not be a problem as only a very small volume is used in the reaction mixture. The enzyme activity of the extract lasts for at least a couple of days, provided it is stored in a refrigerator. 3. To prepare a colorimeter tube, add 2 cm 3 standard pH 7 buffer and 2 cm 3 of 0.1% catechol. Note the time and then add 0.1 cm 3 enzyme extract, quickly mixing the contents Science & Plants for Schools: www.saps.org.uk Polyphenoloxidase assay: p. 1 This document may be photocopied for educational use in any institution taking part in the SAPS programme. It may not be photocopied for any other purpose. Revised 2012. of the tube. Place it into a colorimeter (previously zeroed using a tube with 4 cm 3 water and 0.1 cm3 of the extract). Take readings of the absorbance at regular intervals (e.g. every 10 seconds). 4. Plot a graph of the change in absorbance against time. An increase in absorbance is due to the formation of benzoquinone, the product of the reaction. The initial slope of the graph gives a measure of the polyphenoloxidase activity of the fruit or vegetable extract. Ideas for investigations into the activity of these enzymes in plant tissues the effects of pH, or ionic concentration, or temperature the effects of plant pathogen infection the effects of enzyme inhibitors, such as metal ions the effects of anti-oxidant chemicals Alternative techniques for monitoring the progress of the reaction include a ‘low-tech’ method, such as following the reaction by allowing the formation of melanins overnight, or a ‘high-tech’ method, by following the uptake of dissolved oxygen from a reaction mixture using an oxygen electrode. Science & Plants for Schools: www.saps.org.uk Polyphenoloxidase assay: p. 2 This document may be photocopied for educational use in any institution taking part in the SAPS programme. It may not be photocopied for any other purpose. Revised 2012.