Powerpoint
advertisement

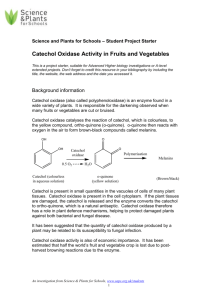
The Sensational Science Behind Antioxidants: Testing Facts vs. Myths of popular antioxidant products Yesterday, you broke up into groups and selected a popular “antioxidant-rich” product to endorse Today chemically test the drinks to see You areyou thewill Research & Development Team and if there’s truth to their marketing. the Marketing Team for your product. Part I: The science behind antioxidants Free radicals cause damage by oxidation Free radical with unpaired electron Victims of Oxidation Peroxide radical O -2 2 Superoxide radical O 2 Molecular Oxygen O2 OO OO Add 2nd e- OO Add 1st e- Add two H+ H OOH H OH H OH Hydrogen peroxide H2O2 Water Add 2H+ Add 3rd e- H O OH Hydroxyl radical OH Hydroxyl ion OH- Add e- H O 4th Hydroxyl ion OH- Free radicals cause damage by oxidation Oxidation occurs when one substance transfers one or more its electrons to another substance + H2O + ½O2 Catechol Benzoquinone Colorless Yellow-Brown “Catechol is oxidized by O2 to become benzoquinone” “Catechol loses its electrons to O2 to become benzoquinone” Phenol Oxidase catalyzes the reaction that changes catechol, in the presence of O2, into benzoquinone + ½O2 Phenol Oxidase + H2O Catechol Benzoquinone Colorless Yellow-Brown Phenol Oxidase catalyzes the reaction that changes catechol, in the presence of O2, into benzoquinone + ½O2 + Catechol Antioxidant! Colorless Phenol Oxidase X + H2O Benzoquinone Yellow-Brown Browning reactions are due to oxidation Phenol Oxidase catalyzes the reaction that changes catechol, in the presence of O2, into benzoquinone + ½O2 Phenol Oxidase + H2O Catechol Benzoquinone Colorless Yellow-Brown Can any of these products inhibit the browning reaction in mushrooms? x 10 Controls: Buffer pH 7 100X Daily Dose of Vitamin C Purifying mushroom oxidase 1. Weigh mushroom 2. Chop mushroom into pieces and put into Ziploc bag 3. Close bag tightly and mash gently with fingers Multiply mass by 4 and add that amount of buffer to the bag Perform the tests! 4. Wet the coffee filter with buffer and place over cup. Use pipette to transfer mushroom extract to filter Spot test Time = 0 min 1. Using the pipette, add two drops of mushroom oxidase to every square on your table. 2. Add the appropriate reactants to each square in the order they are listed Time = 5 min 3. Once all the drops have been added to the 6 squares, start the timer. 4. Observe the reaction for 5 minutes, then answer the following questions. Hint: Use a steady hand so that each drop is the same amount of liquid. Make sure the paper is level, so the drops do not move. Double bonds absorb light http://science-edu.larc.nasa.gov/EDDOCS/Wavelengths_for_Colors.html Light absorption is like a fingerprint for compounds How does a spectrophotometer work? 0.430 Absorbance Readout At Abs Wavelength 490 nm: selector + H2O + ½O2 Light Catechol Colorless 0.240 Prism to disperse light Benzoquinone Yellow-Brown 0.520 Spectrophotometer Test 1. Label your test tubes #2, #4, #6 and buffer blank 2. Add 4 ml of buffer to “buffer blank”. Place the tube into spectrophotometer holder and use the left knob to set the numbers in the absorbance window to 0.000. 3. Add the reactants for Tube #2—use correct pipettes for each reactant! Mix by tapping the test tube, and place tube into spectrophotometer. Start the timer and record absorbance at t=0 min. 4. Remove tube at t = 4:30 min and mix by tapping test tube. Record absorbance at t = 5 min. 5. Repeat steps 3-4 for each Tubes #4, #6. There are many different ways to test for antioxidants Our mushroom oxidase test may not be sensitive enough to pick up on antioxidant levels + ½O2 + Catechol Antioxidant! Colorless Phenol Oxidase X + H2O Benzoquinone Yellow-Brown + ½O2 + Catechol Antioxidant! Phenol Oxidase X Colorless Your drink has antioxidants, but it may be very low amounts compared to 100X Daily Value of Vitamin C + H2O Benzoquinone Yellow-Brown Part II: The marketing behind antioxidants Present a 3 minute marketing campaign that showcases: Your knowledge on the mechanism and health benefits of antioxidants defense against free radicals. Your product’s antioxidant test results. Your opinion why your classmates should buy your antioxidant drink. (Instructor note: show a video clip of antioxidants in the media. In my presentation, I edited together a video of 9 different commercials, interviews on the topic of antioxidants) Edited by Ms. Theresa 1. Do not make scientific claims that you cannot back with evidence 2. Find a different approach to market the product Much research has to be performed before claims can be made Much research has to be performed before claims can be made “I recommend pizza since it is a vegetable and therefore has *tons* of antioxidants.” I’m actually a real doctor! Google me! Sample Ballot Which product is the best antioxidant drink on the market? The best marketing campaign was for: ☐ ☐ ☐ ☐ ☐ ☐ ☐ 7-Up Antioxidant Activate Antioxidants FUZE Triple Berry Lipton Green Tea POM Wonderful Vitamin Water XXX Other ___________ The best scientific argument was made by: ☐ ☐ ☐ ☐ ☐ ☐ ☐ 7-Up Antioxidant Activate Antioxidants FUZE Triple Berry Lipton Green Tea POM Wonderful Vitamin Water XXX Other ___________ Write-in: Based on the overall campaign and facts, I would consider buying: