Enzyme Pre-Lab
advertisement

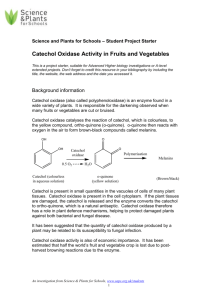
Enzyme Pre-Lab By Adam Wanetik, Spencer Olesky And also Daniel Broadie What are Enzymes? • Enzymes are proteins, each with a unique physical structure • They are “catalysts” which means that they speed up reactions • The shape of each enzyme fits the shape of the reacting molecule for which the enzyme serves as a catalyst Substrate Complex • In an enzyme catalyzed reaction, the reacting molecules are called the substrate. Substrate molecules combine with the enzymes forming a enzyme substrate complex • In this chemical reaction the products are formed and the enzyme is released, and it is unchanged from its original structure Catechol and Benzoquinone • Catechol is a clear organic compound which occurs naturally in trace amounts • Catechol can be found in foods such as apples, bananas and potatoes • Catechol oxidase is the enzyme which catalyzes the color in catechol to turn in into benzoquinone • Benzoquinone (or benzo brown) is what is the brown color that increases as your food ages Catechol and Benzoquinone Form Fits Function • Much like DNA and RNA, the form of the enzyme determines it’s function • For enzymes to function correctly they also need a cofactor/coenzymes • Many vitamins and minerals serve as coenzymes • Substances that interfere with coenzymes or cofactors will also inhibit the enzyme The Lab • In this lab, we will look at the inhibition of enzyme activity by specific chemicals called inhibitors • The inhibitors that we will use is called phenylthiourea (PTU) • Catechol oxidase’s cofactor is copper • PTU can combine with the copper in catechol oxidase to inhibit its enzymatic activity The Lab • Inhibitors affect enzymes in one of two ways: Competitive inhibition takes place when two molecules structurally similar compete for an active site on the molecule • In the experiment we will do, we will find out if PTU is a competitive or non-competitive inhibitor The Lab • We will measure the production of benzoquinone in the presence and absence of the enzyme • We will test this on potatoes both in the presence of the enzyme preincubated with PTU, and preincubated with various concentrations of lemon juice Materials • • • • • • 2 potatoes, peeled and cut into chunks 1 Blender 3 Liters cold distilled water 1 1000-ml beaker 1 Large funnel Several layers of cheesecloth Materials • • • • • • • • • • • Colorimeter Colorimeter cuvettes Distilled water Pipettes Stock solutions of 1% Catechol (Poison!) Enzyme Catechol Oxidase (potato extract) Enzyme Preincubated in Phenylthiourea (PTU) (Poison!) Enzyme Preincubated in pure Lemon Juice (pH = 6.5) Enzyme Preincubated in 1:100 Lemon Juice (pH = 6.5) Enzyme Preincubated in 1:100000 Lemon Juice (pH = 6.5) Procedure • Peel and chunk the potatoes. Put the peeled, chunked potatoes in a blender. Add 700ml of cold distilled water and blend at high speed for 2 minutes. • Line a large funnel with several layers of cheesecloth and place the funnel in a 1000 ml beaker, which has been placed in a container of ice. Filter the potato juice through the beaker Procedure • The filtrate is the potato juice-catechol oxidase extract. It is called "catechol oxidase” throughout the exercises. • Potatoes also contain catechol, so you will need to keep the catechol oxidase extract on ice at all times to retard any natural chemical reaction that might occur. Procedure • Next, we test with different concentrations of the lemon juice (different pH solutions) on the rate of production of Benzoquinone • Make serial dilutions by taking 10 ml of lemon juice to a graduated cylinder and bring to 100ml. Then take 10 ml of that solution and bring it to 10 ml etc. Preparation of Lemon Juice • • • • • • • Concentration Pure 1:10 1:100 1:1000 1:10000 1:100000 pH 2.5 3.2 4 4.8 5.6 6.54 Table 1. Experimental Setup ACHTUNG! • Catechol, hydroquinone, and phenylthiourea are poisons and catechol, in concentration, is a mutagen. Use caution handling these chemicals. Avoid contact with these solutions. If a spill occurs, immediately wipe up the spill with dry paper towels and then use disinfectant solution on the spill site. Dispose of all towels in a designated contamination location. Be sure to notify your instructor of the spill and your cleanup procedures. Your personal safety and the safety of other students is most important. Preparation of Solutions Preparation of 1% Catechol Add 10 grams of Catechol to beaker and bring to 1 liter Preparation of 1% PTU Add 1 gram of PTU to beaker and bring to 100ml Preparation of Lemon Juice Concentrations In this part of the lab we will test the effect of various concentrations of lemon juice (different pH solutions) on the rate of production of Benzoquinone. Lemon Juice is commonly used to preserve fruit. This is because Lemon Juice contains a 5-6% Citric acid solution which will denature the catechol oxidase, thus slowing the oxidation of Catechol to Benzoquinone. Enzyme Concentration Determination • Determine what concentration of Potato extract will catalyze the reaction at such a rate such that the reaction reactions completion in around 5 minutes. • Add 4ml of Catechol to 1 ml of enzyme (so technically no longer a 1% Catechol solution) • Put in Colorimeter and watch reaction proceed. • Make serial dilutions of Enzyme until a “good” rate of reaction is achieved. Standardizing the Colorimeter • Insert water filled cuvette into colorimeter. • Push and hold green button. Colorimeter is now calibrated. • Make sure you colorimeter is set to BLUE Absorption. • Hit run just as you add the final solution to your cuvette and hit stop just as you reach 10 minutes. Standardizing the Colorimeter • Repeat for each run and it will make for a beautiful graph. • Make sure that the cuvette is DRY and free of finger prints Measuring Production of Benzoquinone • • • • Put Catechol into Cuvette Put water into Cuvette Put Enzyme into Cuvette Put Cap on Cuvette, invert 2 times to mix, and place into colorimeter. • Take colorimeter readings every 15 seconds. Measuring Production of Benzoquinone • Remove cuvette from colorimeter every minute, invert twice and immediately return cuvette to colorimeter. • Take readings for 10 minutes. • (note: in the interest of time, skip the 2 control conditions, the teacher will do those) Data Analysis • Graph your raw data • Calculate your maximum rate for each condition.