1 - Pro Pharma Pharmaceutical Consultants, Inc.
advertisement

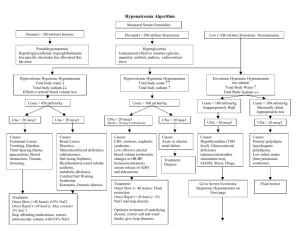
2/12/2016 PRO PHARMA TECHNOLOGY TOOLBOX Generic: Tolvaptan tablets Brand: SAMSCA Strengths: 15mg, 30mg, 60mg 1 INDICATION1 SAMSCA is a selective vasopressin V2-receptor antagonist indicated for the treatment of clinically significant hypervolemic and euvolemic hyponatremia*, including patients with heart failure, cirrhosis, and Syndrome of Inappropriate Antidiuretic Hormone (SIADH) *[serum sodium < 125 mEq/L or less marked hyponatremia that is symptomatic and has resisted correction with fluid restriction] Important Limitations: 1. Patients requiring intervention to raise serum sodium urgently to prevent or to treat serious neurological symptoms should not be treated with SAMSCA. 2. It has not been established that SAMSCA provides a symptomatic benefit to patients. Prevalence: Statistics not available, Incidence: Statistics not available 2 DOSAGE AND ADMINISTRATION1 SAMSCA should be initiated and re-initiated in a hospital. The recommended starting dose is 15 mg once daily. Dosage may be increased at intervals ≥ 24 hr to 30 mg once daily, and to a maximum of 60 mg once daily as needed to raise serum sodium. Monitor serum sodium and volume status. 3 MECHANISM OF ACTION1 Tolvaptan is a selective vasopressin V2-receptor antagonist with an affinity for the V2-receptor that is 1.8 times that of native arginine vasopressin (AVP). Drug Structure1,2 4 ADVERSE REACTION/SIDE EFFECTS 3,4 Most common adverse reactions (≥ 5% placebo) are thirst, dry mouth, asthenia, constipation, pollakiuria or polyuria, and hyperglycemia. P.O. BOX 280130 • NORTHRIDGE, CA 91328-0130 • (818) 701-5438 • FAX (818) 701-0249 2/12/2016 5 TREATMENT GUIDELINES2, 3, 4 Regardless of the patient's volume status, another common feature is to restrict free water and hypotonic fluid intake, since these solutions can only exacerbate hyponatremia. Free water intake from oral intake and intravenous fluids should generally be less than 1–1.5 L/d. Hypovolemic patients require adequate fluid resuscitation from isotonic fluids (either normal saline or lactated Ringer solution) to suppress the hypovolemic stimulus for ADH release. Patients with cerebral salt wasting may require hypertonic saline or normal saline to prevent circulatory collapse; some may respond to fludrocortisone. Hypervolemic patients may require loop diuretics or dialysis, or both, (or other extracorporeal ultrafiltration) to correct the increased total body water and sodium. Euvolemic patients may respond to free water restriction alone. Treatment with Symptomatic and/or severe hyponatremia, in Hypovolemic or Euvolemic state, generally requiring hospitalization for observation, careful monitoring of fluid balance and weights, and frequent sodium checks. Any inciting medications should be discontinued if possible. There is no consensus about the optimal rate of sodium correction in symptomatic hyponatremic patients. A reasonable rate is 10–12 mEq/L/d in mildly to moderately symptomatic patients. A more aggressive rate of 1–1.5 mEq/L/h (to a maximum correction of 10–12 mEq/L) has been used in severely symptomatic patients. Sodium concentration should be monitored as frequently as every 1–2 hours. As the symptoms improve or resolve, the correction rate should be reduced to roughly 0.5 mEq/L/h. To avoid overcorrection, the physician should stabilize the sodium in the range of 125 to 130 mEq/L. In severely symptomatic patients, the physician should calculate the patient's sodium deficit and deliver hypertonic saline (ie, 3% saline) at the appropriate rate. In general the saline infusion rate should be approximately 0.5 mL/kg body weight/h; rates of 1 mL/kg/h or greater may represent a miscalculation of the sodium deficit or mathematical error. Hypertonic saline in hypervolemic patients can be hazardous, resulting in worsening volume overload, pulmonary edema, and ascites. 1. Demeclocycline (300–600 mg orally twice daily) is used for patients who cannot adequately restrict water restriction or have an inadequate response to conservative measures. It inhibits the effect of ADH on the distal tubule. Onset of action may require 1 week, and urinary concentrating ability may be permanently impaired, resulting in nephrogenic diabetes insipidus and even hypernatremia. Demeclocycline may be more nephrotoxic in patients with cirrhosis. 2. Vasopressin antagonists may revolutionize the treatment of euvolemic and hypervolemic hyponatremia, especially in persons with heart failure such as lixivaptan, tolvaptan, and satavaptan, which are selective vasopressin-2 receptor antagonists. V2 receptors primarily mediate the diuretic effect of ADH. For hospitalized patients with euvolemic SIADH, conivaptan is given as an intravenous loading dose of 20 mg delivered over 30 minutes, then as 20 mg continuously over 24 hours. Subsequent infusions may be administered every 1–3 days at 20–40 mg/d by continuous infusion.* * Cho Kerry C, Fukagawa Masafumi, Kurokawa Kiyoshi, "Chapter 21. Fluid & Electrolyte Disorders" (Chapter). McPhee SJ, Papadakis MA, Tierney LM, Jr.: CURRENT Medical Diagnosis & Treatment 2009: http://www.accessmedicine.com/content.aspx?aID=10909. P.O. BOX 280130 • NORTHRIDGE, CA 91328-0130 • (818) 701-5438 • FAX (818) 701-0249 2/12/2016 6 PRICING (relative) 5 7 Drug Comparison1 http://www.accessdata.fda.gov/drugsatfda_docs/l abel/2009/022275lbl.pdf http://www.drugbank.ca/drugs/DB00872 http://www.accessdata.fda.gov/drugsatfda_docs/l abel/2008/021697s001lbl.pdf Conivaptan is Tolvaptan is a nonpeptide, dual vasopressin V1A and V2 receptors antagonist. a selective vasopressin V2-receptor antagonist. Starting dose begin with a loading dose of 20 mg IV administered over 30 minutes, followed by 20 mg of conivaptan administered in a continuous intravenous infusion over 24 hours. Following the initial day of treatment, conivaptan is to be administered for an additional 1 to 3 days in a continuous infusion of 20 mg/day. Starting dose is 15 mg once daily. Dosage may be increased at intervals ≥ 24 hr to 30 mg once daily. Maximum dose is 60 mg once daily as needed to raise serum sodium. Tolvaptan affinity for the V2-receptor is 29 times greater than for the V1a-receptor. Maximum dose (after the loading dose) is 40 mg per day. The predominant effect of conivaptan is through its V2 antagonism. 1. Drugs@FDA : http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/ 2. DrugBank: http://www.drugbank.ca/ 3. Dipiro: USC Pharmacology Website P.O. BOX 280130 • NORTHRIDGE, CA 91328-0130 • (818) 701-5438 • FAX (818) 701-0249 2/12/2016 4. WebMD: http://www.webmd.com/ 5. DrugStore: http://www.drugstore.com/ 6. Rxfiles: http://www.rxfiles.ca/rxfiles/modules/druginfoindex/druginfo.aspx 7. Rphworld: http://www.rphworld.com/pharmacist/link-347.html 8. Rphworld: http://www.rphworld.com/pharmacist/viewlink-25534.html 9. CenterWatch: http://www.centerwatch.com/drug-information/index.htm P.O. BOX 280130 • NORTHRIDGE, CA 91328-0130 • (818) 701-5438 • FAX (818) 701-0249