Grade 10 Academic Science – Chemistry
advertisement

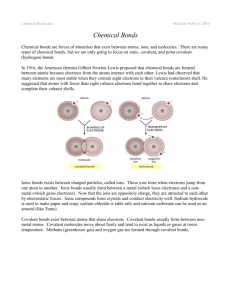
Grade 10 Academic Science – Chemistry Covalent Bond Covalent Bond Covalent bonds are forces that hold atoms together. The forces are formed when the atoms of a molecule share electrons. The bonds are strong. The exception is hydrogen bonds (see below). There are two types of covalent bonds: (1) Polar Covalent and (2) Non-polar Covalent. In a non-polar covalent bond, the electrons are shared equally by the adjacent atoms. As a consequence, there is NO CHARGE SEPARATION between atoms. Since there is no charge separation in the covalent bonds, this molecule cannot enter into a charge interaction with water. Thus, a non-polar covalently—bonded molecule is hydrophobic (i.e., cannot form hydrogen bonds with water). These molecules are primarily hydrocarbons composed of hydrogen and carbon atoms. Other examples are fatty acids, phospholipids and cholesterol. In a polar covalent bond, the electrons are NOT shared equally by the atoms. Rather, the shared electrons spend a greater amount of time closer to one nucleus than the other nucleus. In the example of water, the shared electrons are closer to the oxygen nucleus than the hydrogen nucleus. Why? There is a significant difference in the electron affinity between the oxygen and hydrogen. As a result, the oxygen has a partial negative charge and the two hydrogen have a partial positive charge. NOTE: This is not an ionic bond because the electrons are shared, Instead, there is a simple charge separation. Peptides and amines are also polar covalent. Biologically, polar covalent molecules form weak hydrogen bonds – a bond formed when a charged part of a molecule forms an electrostatic interaction with a substance of opposite charge (positive attracted to negative). As a result, they degrade or break apart easily and they are hydrophilic (i.e., form a charged interaction with the water molecules).