Diels-Alder Synthesis: Lab Manual
advertisement

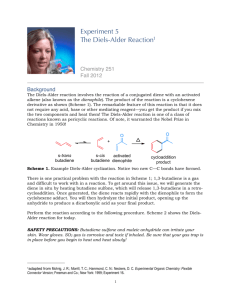
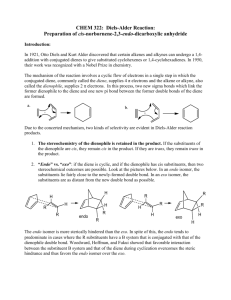
CHEM 334L Organic Chemistry Laboratory Revision 4.0 A Diels-Alder Synthesis of cis-Norbornene-5,6-endo-Dicarboxylic Anhydride In this laboratory exercise we will synthesize the compound cis-Norbornene-5,6-endoDicarboxylic Anhydride; a compound which, in and of itself, is of relatively little importance. However, the synthetic method used to generate this compound, the Diels-Alder Reaction, is extremely important. The Diels-Alder Reaction, named after the German chemists Otto Diels and Kurt Alder whose experiments first demonstrated the nature this reaction type, is considered to be one of the few truly new Organic reactions of the 20th century. Otto Diels (http://www.uni-kiel.de/ps/cgi-bin/ fo-bio.php?nid=diels&lang=e) Kurt Alder (http://en.wikipedia.org/wiki/File:Kurt_Alder.jpg) P age |2 In the Diels-Alder Reaction, a conjugated diene will react with a dienophile, if an appropriate electron-withdrawing group is attached to the dienophile, to produce a carbocycle containing two new Carbon-Carbon bonds. This is important because the synthetic formation of Carbon-Carbon bonds is difficult and because the products of Diels-Alder Reactions contain cyclohexene rings that can be used to form complex molecular structures. The Diels-Alder reaction is a special case of the more general class of cycloaddition reactions between (Pi) systems, the products of which are called cycloadducts. In the Diels-Alder reaction, an assembly of four conjugated atoms containing four electrons reacts with a double bond containing two electrons. Due to this, the reaction is called a [4 + 2] cycloaddition. The mechanism for the reaction can be thought of as a concerted movement of three electron pairs; illustrated below for the reaction of 1,3-Butadiene with Ethene. (A word of caution is in order here; the mechanism for this reaction is better described using Molecular Orbital Theory. This is discussed in the attached Appendix.) Note the diene is in an s-cis configuration. This is required for the cycloaddition to proceed. Dienes in the s-trans configuration are not configured such that the concerted movement of the electrons can proceed easily. Electron-withdrawing groups, denoted as X in the first example, attached to the dienophile help activate the reaction. This is because they create an alkene which is an electron-poor target for the concerted attack of the electron-rich diene. Groups such as -CF3 withdraw electrons inductively, whereas groups such as the carbonyl group withdraw electrons by resonance. P age |3 Our desired product can be produced by a Diels-Alder reaction between Cyclopentadiene and Maleic Anhydride: Cyclopentadiene makes for a particularly facile diene because it is locked into the s-cis configuration by the bridging -CH2- group. Additionally, Maleic Anhydride is strongly activated by the two -C=O fucntionalities. Therefore, our Diels-Alder reaction will proceed without much coaxing. As a consequence of the concerted mechanism of the Diels-Alder reaction, the reaction is stereospecific. For example, reaction of 1,3-Butadiene with dimethyl cis-2-butenedioate gives dimethyl cis-4-cyclohexene-1,2-dicarboxylate. On the other hand, dimethyl trans2-butenedioate yields the trans product. P age |4 (Question: Is this an issue for our particular reation?) Additionally, Diels-Alder reactions are highly stereocontrolled with respect to the orientation of the starting materials relative to each other. The reaction of Cyclopentadiene with dimethyl cis 2-butenedioate, for instance, can form two different products. One in which the two ester substituents on the bicyclic frame are on the same side as the methylene bridge (exo product), the other in which they are on the opposite side (trans) of the bridge (endo product). Exo substituents are placed cis with respect to the shorter bridge; endo substituents are positioned trans to this bridge. The Diels-Alder reaction usually proceeds with endo selectivity. This means that the product in which the activating electron-withdrawing group of the dienophile is located in the endo position is formed faster than the alternative exo isomer. This happens even though the exo product is sometimes more stable than the corresponding endo product and is due to a variety of steric and electronic influences on the transition state of the reaction. Thus, we can expect the reaction between Cyclopentadiene and Maleic Anhydride to form the endo product. Finally, Cyclopentadiene, is obtained from a light oil derived from coal tar distillation. Because Cyclopentadiene is such a reactive system, at Room Temperature it exists as a stable dimer, Dicyclopentadiene. This is a Diels-Alder adduct of two molecules of the diene. Thus, generation of Cyclopentadiene involves heating the dimer to initiate a "retro-" or "reverse-Diels-Alder" reaction. P age |5 So, our Diels-Alder synthesis of cis-Norbornene-5,6-endo-Dicarboxylic Anhydride will first involve the "cracking" of the Dicyclopentadiene into Cyclopentadiene. This product will then be immediately treated with Maleic Anhydride to carry-out our desired DielsAlder reaction. P age |6 Pre-Lab Questions 1. What other reactions that we have studied, in CHEM 333L, involve Carbon-Carbon bond formation? 2. Classify each of the following alkenes as electron poor or electron rich, relative to ethene. Explain your assignments. 3. Add structures of the missing products or starting materials to the following schemes: P age |7 Procedure Cracking the Dicyclopentadiene This procedure may be performed collectively by the Laboratory Instructor as the diene is particularly noxious. 1. Measure 20 mL of Dicyclopentadiene into a 100 mL round bottom flask and arrange for fractional distillation into an ice-cooled receiver. 2. Heat the dimer with a heating mantle until it refluxes briskly and at a rate such that the monmeric diene begins to distill in about 5 minutes and soon reaches a steady boiling between 40oC and 42oC. Do not exceed the boiling point of 42oC. Synthesis of Norbornene 1. In a fume hood, place 6g of Maleic Anhydride in a 125 mL Erlenmeyer flask and dissolve it in 16 mL Ehtyl Acetate by heating on a hot plate. (Always wear gloves and handle the Maleic Anhydride in a fume hood. If the Malein Anhydride is supplied in briquette form, powder it using a mortar and pestle. Wear goggles, gloves and a lab coat when doing this.) 2. Add 16 mL of Ligroin (bp 60oC-80oC) or Hexane, cool the solution thoroughly in an Ice-Water bath, and leave it in the bath. (Some Anhydride may crystallize.) 3. If the freshly distilled Cylcopentadiene is slightly cloudy because of moisture condensation in the cooled receiver, add 1g of Calcium Chloride pellets to remove the moisture. 4. Measure 6 mL of dry Cyclopentadiene and add it to the ice-cold solution of Maleic Anhydride. Swirl the solution in an ice bath for a few minutes until the exothermic reaction ceases and the adduct separates as a white solid. 5. Heat the mixture on a hot plate until the solid is completely dissolved. Allow the solution to stand undisturbed while crystallization proceeds.† 6. Using a short-stem funnel, filter off the crystals. 7. Obtain an melting point for the product. 8. Determine your percentage yield. P age |8 † If moisture has gotten into the system, a little of the corresponding Diacid may remain undissolved. If this occurs, the Diacid should be removed by filtering the hot solution. Spectroscopy 1. Obtain an IR spectrum of the product. (Consult with your Laboratory Instructor about how to do this.) 2. Compare your spectrum with that published in the Appendix. Rationalize all the "identified" IR peaks in the published spectra of the product and the Dicyclopentadiene. 3. Obtain an NMR spectrum of the product. (Consult with your Laboratory Instructor about how to do this.) 4. Assign all the NMR peaks in the spectrum. P age |9 Post-Lab Questions 1. What is the purpose of adding the Ligroin or Hexane in step #2 of the Synthesis procedure? 2. Look-up the "12 Principles" of Green Chemistry. (wikipedia anyone?) Which of these principles are conformed with by our synthetic method? Which of these principles are explicitly violated? 3. In step #6 of the Synthesis, why do we use a short stemmed funnel rather than one that has a long stem? 4. When cracking the Dicyclopentadiene, why do we not want to exceed a boiling point of 42oC? 5. The Diels-Alder reaction can also occur in an intramolecular fashion. Draw the two transition states leading to products in the following reaction: P a g e | 10 Appendix - Published IR Spectra cis-Norbornene-5,6-endo-Dicarboxylic Anhydride Dicylcyclopentadiene Spectra taken from: A Diels-Alder Synthesis; Organic Chemistry Laboratory Manual; Portland Community College; 2005. P a g e | 11 Addendum A Molecular Modeling Exercise This Addendum will be delivered and performed at a later date.