Genetically Modified Organisms: Who is growing them? Do They
advertisement

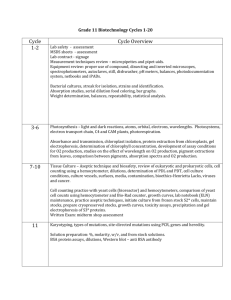
File: project4.doc 2/12/2016 Steinmaus Project Genetically Modified Organisms: 4 Who is growing them? Do They Move? Background Biotechnology has allowed scientists to genetically engineer crops to be herbicide resistant (Roundup Ready ®), to produce their own insecticide (endotoxin from Bt), to be more nutritious (Golden Rice), to be resistant to pathogens (programmed cell death: Caspase3 Inhibitor DEVD, Xa21 Gene). And these are just a few examples. Undoubtedly, biotechnology, specifically genetically modified organisms (GMOs), will be a major force in the future of agriculture. Already, 61% of the cotton acreage, 54% of the soybean acreage, and over 20% of the corn acreage has been planted to genetically modified varieties that are herbicide resistant. In 2003, 167 million acres of crop land was planted to a genetically modified, or transgenic, variety. velvetleaf not sprayed Roundup sprayed to here Roundup Ready® soybean untransformed soybean Concerns: 1. However, there has been much controversy with genetically modified organisms (GMO) to the point that governments around the world have prohibited the planting of GMO crops in their countries. Major food companies go to great lengths to insure that GMOs are not in their foods. Scientists assure consumers that no significant unintended effect has been detected in birds and mammals that were fed commercialized transgenic crops. Biotech companies submit to the U.S. EPA (Environmental Protection Agency) a “no observed effect concentration” (NOEC) for birds and mammals. Purified genetically modified proteins must be added to test diets because ground up GM crop material contains far below the NOEC. In other words, they have to spike test feed with GM proteins to get ANY kind of effect. page 1 of 11 File: project4.doc 2/12/2016 Steinmaus 2. An additional concern for biotech companies is to recoup the costs of their investment into research and development. It will cost a biotech company millions of dollars to develop a significant GMO. They must recoup the costs of this research and development (R&D) if they are to stay in business. Seeds are the vehicle by which GMO crops are distributed. Growers must buy this GMO seed and sign a contract that they will not retain a portion of this year’s harvest to plant next year’s crop. It is common for growers to hold a small portion of their crop for planting in the subsequent year. Unfortunately, if a grower purchases and likes the GMO system s/he must repurchase a new batch from the biotech company any year they plan to use the GMO system. Otherwise, the company would have to charge extremely high prices for that first batch of seed in order to recoup the costs associated with research and development. 3. A third concern has to do with transgenes from the GMO crop moving to closely related plants including weeds and other non-GMO crops of the same species (Roundup Ready ® cotton to regular cotton) being grown in the area. It is clear that gene flow does occur in certain species. Corn pollen can theoretically travel 180 km, however, 200m is considered a safe isolation distance to prevent crosspollination. In fact a grower in Canada claims that his canola crop tested positive for GMO because the pollen from an adjacent canola field, which was GMO blew into and pollinated his canola. Check out the following: Percy Schmeiser, the Saskatchewan farmer made news around the world in his fiveyear fight against Monsanto, whose Roundup Ready crop varieties are used by thousands of farmers in Canada and around the world. Monsanto took Schmeiser to court after it discovered its patented transgenic canola growing on his farm. Schmeiser had not signed a C$15 (US$9.60)-per-acre agreement with the company to grow the canola, which is genetically modified to tolerate the Roundup herbicide, facilitating weed control. At his trial, Schmeiser said that he had spent 40 years cultivating his own, conventional canola varieties, saving seed from one crop to plant the next. Schmeiser also testified that he did not know how the genetically modified plants ended up on his farm. He has suggested that seeds blew off passing trucks, or pollen from nearby farms was carried in by wind, insects, or birds. Last March, a Federal Court judge ruled that Schmeiser knew or ought to have known that he had saved and planted seed that was Roundup tolerant. Justice Andrew MacKay also found that farmers can generally own the seeds or plants grown on their land if they are blown or carried in from elsewhere — but not when it comes to genetically modified seed. MacKay also ordered Schmeiser to pay Monsanto court costs of C$153,000 (US$98,000) as well as the profits from his 1998 canola crop, worth about C$20,000. The ruling, however, did little to dissuade Schmeiser from his cause. "It's such an important issue now, in regards to the patenting of life-giving forms, so my case now is not only a Percy Schmeiser case now, it's a case for farmers and indigenous growers and people throughout the world,'' Schmeiser said. See: http://www.enn.com/news/wire-stories/2002/05/05162002/reu_47238.asp page 2 of 11 File: project4.doc 2/12/2016 Steinmaus The real question is whether Schmeiser used glyphosate on his canola because that would be the only way to benefit from the Roundup Ready® system. There were no varietal advantages to the Roundup Ready ® canola except that they could be sprayed with Roundup ®. Problem for Lab: As rumor would have it, Dr. Phillips, our organic agriculture expert and Community Serviced Agriculture (CSA) mentor, has been planting genetically modified crop species. Specifically, the word is out the he may be using Roundup Ready ® cotton. When asked for a statement on the accusation, Dr. Philips quipped, “no comment”. Now, who could blame him, he is only one man, and there is so much weeding to do that Roundup Ready ® (RR) offers the clear solution to Dr. Phillips dilemmaso many weeds and only one hoe! Now in order to put Dr, Phillips away we need to be sure he’s got Roundup Ready ® cotton. We can’t just rely on empty RR seed bags…we need real evidence, we need DNA evidence! It is important that Dr. Phillips not know what we are up to because he’d be on the first plane to South America or wherever these Frankenfood people go (certainly not Canada). So mum’s the word. The suspected GMO cotton field sowed by Dr. Phillips Objectives With the completion of this project you will be able to: understand some of the basic techniques used by molecular biologists determine whether an unknown plant sample is genetically modified or not. page 3 of 11 File: project4.doc 2/12/2016 Steinmaus have an idea of the possibility that transgenes from GMO cotton could move to a non-GMO cotton. This will also give you some idea to the degree to which cotton outcrossesfor this first year, the only potential for outcrossing that we’ll see is if pollen from the Roundup Ready ® cotton made it onto a leaf of non-GMO cotton. study the morphology of cotton plants and flowers so that we can better understand the freedom to outcross with other plants Materials and Methods Materials DNA Isolation BIO 101 FastDNA Kit. Contains all the necessary buffers and reagents for DNA isolation. micropipettors: Rainin P10, P20, P100, P1000 heating block microfuges (14000 rpm) 2ml tubes plastic bags for sampling Polymerase Chain Reaction (PCR) thermocycler micropipettors: Rainin P10 primer set: 2 small (10-20 bases) complementary strands of DNA designed to detect the Cauliflower Mosiac Virus (CaMV) which is used in Roundup Ready ® cotton Gel Electrophoresis agarose TAE buffer PCR products (from previous step) gel boxes with power supply ethydium bromide stain Gel Documentation Station (to see DNA bands on gel under UV light) Methods A CRSC 131 student isolating DNA from his cotton sample. Notice how he is doing a great job because he is following the detailed instructions at each station. If he has a question, he asks firstthen takes action. page 4 of 11 File: project4.doc 2/12/2016 Steinmaus DNA Isolation: follow and write down the protocol that is set up for you in lab. Essentially it will include: 1. Sample cotton: collect about 200mg worth (a leaf or two). Get young tissue. Label the row number from where you sampled on the bag and in your lab notebook. 2. Bring sample back to lab. Label EVERYTHING (tubes) with your initials and row# as you go along. 3. Weigh out ~200 mg leaf tissue on a scale and put into the tube with Lysing Matrix, which is a ceramic bead and garnets (sand like). Add the called for amount of buffer. 4. Homogenize in the Fast Prep machine (We call it a paint shaker). As this machine shakes the tube, the plant sample is pulverized as the bead and garnets bang together. This will break open the cells releasing their DNA. Plant cells are tough because they have a cell wall. 5. Centrifuge so that the debris (busted cell parts) forms a pellet at the bottom of the tube. The DNA will be in the liquid portion. 6. Transfer the most of the supernatant (the liquid portion, not the pellet) to a clean tube. 7. Add Binding Matrix mix GENTLY. Centrifuge and discard the supernatant. The DNA is bound to the clay particles (i.e. the binding matrix). Resuspend the pellet with SEWS-M. Spin in centrifuge again, discard the supernatant again. DNA is still on clay matrix. Spin again, discard any supernatant that might be left. 8. Elute the DNA from binding matrix by GENTLY resuspending in DES then incubate for 2-3 minutes. This will cause the DNA to unbind from the matrix and mix in with the liquid portion. 9. Spin in centrifuge, transfer supernatant (which contains the DNA) to a new tube. Do not include any of the pellet. Be GENTLY here so that you do not shear any DNA. Label your tube with initials and row#. Polymerase Chain Reaction (PCR) Now that we have the DNA from the cotton, so what? How do we whether Dr. Phillips is going down or not? If we put our DNA in a gel and ran electricity from one side to the other the DNA would move. This is called electrophoresis. The problem is we need a way to tell Roundup Ready ® DNA from non-GMO cotton. To do this we are going to amplify a portion of the Roundup Ready® gene. This is a technique will make more than a billion copies of a portion of our Roundup Ready® gene. By having a billion copies, we will be able to see those on a gel as a distinct band corresponding to a length of 200 bases of DNA. It will be 200 bases long because we are using two small DNA primers that will bind to either side of the Roundup Ready ® gene with a gap of 200 bases between them. The rest of the DNA will be much longer (1000’s of bases long) and lag way behind the amplified Roundup Ready® gene. Because we are using only DNA primers that correspond to the Roundup Ready® gene, if you have non-GMO cotton you will not see a band of DNA that is 200 bases long. You will see a big blob of unamplified DNA 1000’s of bases long, however. We will talk more about PCR in lab page 5 of 11 File: project4.doc A student loading the PCR thermocycler. 2/12/2016 Steinmaus PCR amplifies the DNA portion between two primers. One cycle is shown here30 cycles will result in >billion copies of that portion of DNA. Follow the directions in lab, but essentially all you’ll need to do is: 1. Pipette some of the primer mix (usually about 15μl) into a PureTaq Ready-to-go PCR Beads tube. The primer mix will contain the primers designed to amplify ONLY a 200 base portion of the Roundup Ready® gene, a bunch of single A, T, C, and G’s, and a special DNA polymerase called Taq polermase, and any appropriate bufferLABEL your tube with row# and initials. 2. Pipette some of your isolated DNA (usually about 10 μl) into the PCR tube. 3. Place your Ready-to-go PCR tube into the PCR tray (write the row number and column letter from the PCR tray for your tube in your lab notebook). 4. Place tray into PCR thermocycler. 5. Turn on the thermocycler, select the appropriate program (probably I’ll name it CRSC 131)”run” 6. After a few hours the cycling (i.e. amplification) will be complete. The samples can now go to the freezer before electrophoresis. Gel ElectrophoresisWEAR GLOVES AND EYE PROTECTION When agarose is melted and cooled in water it will form a gel due to hydrogen bonding. Your isolated DNA will move in the gel toward the anode (+) because it is negatively charged. Smaller pieces move faster than larger pieces. There is a linear relationship between the log of mobility and gel concentration over a certain range of DNA fragment sizes. Therefore, we’ve optimized the concentration of agarose to separate out a 200 base fragment (which will come from those samples that contain the Roundup Ready ® gene) from the rest of the isolated DNA. page 6 of 11 File: project4.doc 2/12/2016 Steinmaus 1. prepare a 2% agarose gel: 0.2 grams of agarose with 20 ml of TAE buffermicrowave on high for 30-40 secondsallow to cool. 2. Get your gel boxes ready to receive the molten agarose (actually, it should be cool enough to touch the flask) by placing the tray, dams and comb into their appropriate slotspour the agarose into the tray, allow to hardenpull out the dams and the combadd TAE buffer to the gel box slowly so that the level just overtops the gel and fills the anode and cathode basin. 3. Obtain your PCR reaction tube from the PCR trayadd 5μl of loading buffer (a dye so that you can see the progression of DNA during electrophoresis) and mix. 4. Each student in the group sharing a gel box should load 5 μl of PCR/loading buffer mix to a well in the gel (the well is the indentation left by the comb after it was pulled) 5. Secure the gel box lid and plug into the power supplyset power to 85voltspress start. 6. Watch the loading buffer dye as it migrates across the gel toward the anode (with the DNA)turn off when about ¾ of the way across. 7. Wearing eye protection and gloves, transfer the tray with the gel to the ethidium bromide tanks. page 7 of 11 File: project4.doc 2/12/2016 Steinmaus Student loading an el;ectrophoresis gel with DNA after PCR amplification Gel Documentation. WEAR GLOVES AND EYE PROTECTION DNA will bind tightly to ethidium bromide which illuminates brightly under UV light. So we will stain the DNA with ethidium bromide and then take a look under UV light. 1. Using a gloved finger, slide the gel from the tray into the ethidium bromide solutionlet sit for 10 minutes. 2. Use a spatula to transfer the gel to waterlet sit for 10 minutesthis will diffuse any unbound ethidium bromide for a clearer picture. 3. Still using gloves and eye protection, use a spatula to transfer the gel to a GEL TRANSFER container. 4. Using the Gel Doc station, we will capture and print the image of your gel for your further analysis. known controls + direction of DNA movement: small fragments will be on right, larger on left cotton RR cotton cotton RR cotton cotton known controls + 200 base pairs= Roundup Ready® page 8 of 11 File: project4.doc 2/12/2016 Steinmaus Timeline Week 1. Isolate DNA and begin PCR. Instructor will store your PCR products in freezer for next week Week 2. Run PCR products on gel electrophoresis, document, exchange information for your table, and interpret results. Results Observations 1. When you have time (either after we do the DNA isolation or for homework outside of lab) take note of any differences between the cotton plants in different rows. Characteristics to look at are: leaf shape, size or height, color, leaf texture. Construct and fill in a table like the one below in your lab notebook. Row # (circle your row#) leaf shape leaf color size 1 2 3 4 5 6 7 8 page 9 of 11 height leaf texture flower boll File: project4.doc 2/12/2016 Steinmaus 2. Record the results of the PCR and electrophoresis in your lab notebook. Put a copy of the printout in your notebooks. Look at the gel doc printouts. You should be able to see the wells into which you injected the PCR products. DNA will move from the wells toward the far end of the gel away from the wells (toward anode). Large pieces move slower than small pieces through the gel. The individual A, T, C, and G’s that were left over in the PCR will travel the fastest. Then the unused primers, then the amplified CaMV, and then the intact genomic DNA strands left over from the DNA isolation. Construct and fill in a table like the one below in your lab notebooks. We will exchange information amongst the class in coming weeks. Row # (circle your row#) DNA (>1000 bases) dNTPs and primers (1-30 bases) 1 2 3 4 5 6 7 8 page 10 of 11 CaMV (200 bases) actual (get from instructor) File: project4.doc 2/12/2016 Steinmaus Discussion Be able to discuss the following questions using the results from our project. Put your answers in your lab notebook. 1. Can you tell any differences between the rows just by looking at them? Can you tell which cotton is genetically modified (Roundup Ready ®)? How? 2. How would you interpret a gel that shows a band corresponding to about 200 DNA bases long? 3. How would you interpret a gel that nothing around the 200 base length? 4. How would you interpret a gel that was blank? Would you say it was not Roundup Ready®? What next? 5. What does it mean if you saw shadows or bands corresponding to about 1-20 bases? 6. How about a thick band that formed a short distance from the well you injected with your PCR product? 7. What situations can you come up with where it would be important to know whether an unknown cotton sample was Roundup Ready ®? 8. Why is it important to know whether a non-Roundup Ready® cotton plant that was growing next to Roundup Ready ® cotton tested positive for CaMV? 9. What is a very simple way for us to tell if an unknown cotton plant was Roundup Ready ® of not? page 11 of 11