Atomic Number and Mass Number
advertisement

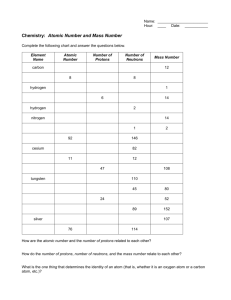
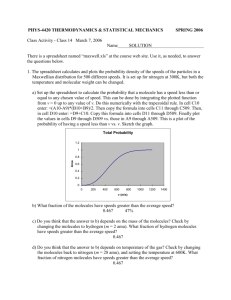
Chemistry: Atomic Number and Mass Number Name: Complete the following chart and answer the questions below. Element Name Atomic Number Number of Protons Number of Neutrons Mass Number carbon 6 6 6 12 Oxygen 8 8 8 16 hydrogen 1 1 0 1 Carbon (carbon 14) 6 6 8 14 Hydrogen (tritium) 1 1 2 3 nitrogen 7 7 7 14 Hydrogen 1 1 1 2 Helium 2 2 2 (1 is also ok) 4 (3) Calcium 20 20 20 40 How are the atomic number and the number of protons related to each other? Atomic number = number of protons How do the number of protons, number of neutrons, and the mass number relate to each other? # of protons + # of neutrons = mass # What is the one thing that determines the identity of an atom (that is, whether it is an oxygen atom or a carbon atom, etc.)? # of protons Match the terms (The definitions have been moved next to their terms) A. polar molecules molecules that possess distinct poles B. hydrophilic C. capillary action D. cohesion E. solute molecules attracted to water and readily dissolve attraction of water molecules to each other and to another that is strong enough to overcome the force of gravity (yes I changed it) attraction of water molecules to each other through hydrogen bonds material that dissolves in a solvent F. high specific heat G. adhesion H. solvent ability of a molecule to absorb a lot of heat before its temperature rises attraction of water molecules to any hydrophilic substance dissolves a solute I. homeostasis maintaining constant conditions J. nonpolar molecules K. hydrophobic molecules that lack regions of electric charge repelled by water molecules L. cooling agent ability to cool a surface during evaporation M. surface tension N. solution a film or layer at the surface of molecules that resists being separated resulting product of a solvent and solute O. hydrogen bond P. universal solvent when oppositely charged parts of molecules are attracted to each other water’s ability to dissolve many substance