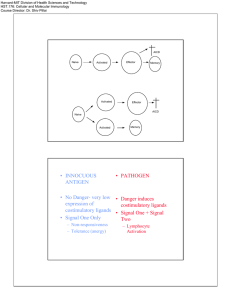
Microbiology Brian Bolerjack Activation of B and T cells 48 minutos
advertisement

Microbiology Activation of B and T cells 09-03-08 Brian Bolerjack 48 minutos What do we mean by lymphocyte activation in general terms? Lymphocytes are quiescent when generated in bone marrow and thymus, migrate into the blood and are transiently homing in the spleen or other immune organs. They are in the G0 state, not actively metabolizing. They are waiting to get the signals they need to enter the cell cycle, that leads to proliferation and differentiation. When you start with T or B cells, the initial signal required to signal the activation response, signal 1, is Ag, which will bind to the AgR on the T or B cell. That triggers the initiation of protein synthesis. You will have entry into the cell cycle and proliferation. This is important because it leads to clonal expansion, so you generate a large number of daughters that all have the same Ag specificity as the original B or T cell. You increase the number of cells that can respond to a particular pathogen, and this is important in the adaptive immune response. Also, protein synthesis, gene transcription, entry into cell cycle and proliferation, will lead to differentiation of the cell into the effector cells that carry out the immune response. The first signal that is Ag is sufficient to induce signal transduction that will lead to protein synthesis, and alteration in gene transcription. T and B cells will then need more signals to undergo proliferation and differentiation. You must have a 2nd co-stimulation signal and cytokine signals for optimal proliferation and differentiation. When he says lymphocyte activation, he ultimately means all the way to differentiation into an effector cell, which requires a signal through the AgR and the co-stimulator receptor on the T or B cell. That’s an important take home point to remember. 1: Ag receptors on T and B cells. On the B cell the AgR is membrane immunoglobulin. On the average naïve B cell, that can be membrane IgM or IgD. They will differ in the heavy chain constant region, which confers the isotype. On any given B cell all the receptors, whether IgM or IgD, will share the same heavy and light chain variable regions which pair up to confer Ag specificity. So all membrane Ig will have the same Ag specificity. 2: When you look at the variable heavy or light domain and stretch it out to the 110-120 AA sequence and look at variability in position, there are areas of hypervariability. For both variable heavy and light chains you have three regions of hypervariability. Then there are regions of constants, called framework regions, which confer the 3D structure of the Ag receptor. The hypervariable, or complementary (?) determining regions 1,2, and 3, when you pair the light chain and heavy chain together, each one contributes 3 complementary determining regions. The total of the six hypervariable regions is what determines the Ag specificity for the B cell Ag receptor. 3: When you look at the three dimensional globular structure of the AgR on a B cell, the hypervariable regions, Ag binding sites, are all positioned at the amino terminus, and are situated to form an Ag binding site. It can be flat or concave or what have you, and provide specificity for interaction with Ag. 4: The membrane bound receptor, Ig, is important to recognize Ag, but that’s all it can do. It can’t transducer a signal to the cell to induce activation. The membrane Ig, consisting of two heavy and two light chains disulfide bonded together, will contact Ag, but it is the associated heterodimer that can send the signal into the cell and alter gene transcription. The membrane receptor is noncovalently associated with a heterodimer, which is itself disulfide bonded together and consists of an alpha and beta chain. They are sometimes referred to as Ig-alpha and Ig-beta. Microbiology Brian Bolerjack Activation of B and T cells 48 minutos 09-03-08 It is also known as CD79A and CD79B, and it is important for a few things. When the heterodimer noncovalently binds to membrane Ig, it is responsible for bringing it to the surface of the cell. Without the heterodimer the Ig cannot reach the surface of the cell. The second thing it does is transduce the signal, which it does through the cytoplasmic polypeptide motifs ITAM- Immunoreceptor Tyrosine-based Activation Motif. In terms of the AA sequence, there is a tyrosine, two variable AA’s, a Leucine/Isoleucine, 6-9 variable AA’s in the middle, and then it is repeated. What is important about this is that when Ag binds to the membrane Ig, this will cause redistribution of the AgR complexes in the membrane of the B cell, so the entire AgR complex is brought into proximity of tyrosine kinases, called SARC (?) family kinases, and those kinases will phosphorylate the tyrosine residues on the alpha and beta polypeptides. Once the motifs are phosphorylated, that enables them to recruit other signaling proteins from the cytoplasm up to the membrane and localize those proteins so they can initiate and propagate signal transduction. That’s the basic mechanism involved in the initial activation of the B cell when it encounters Ag. 5: The T cell Ag receptor. There are commonalities and differences. On T cells you have two peptides that make up the TCR that are disulfide bonded together. So it is structurally different than the B cell AgR. In common is at the amino terminus you have variable domains. So you have two constant and two variable domains. 6: These variable domains are encoded by variable gene elements that confer within the 110-120 AA domain three hypervariable regions for both the alpha and beta variable domain. So you have CDR 1,2 and 3 within the variable domains. As is the case for the B cell AgR, the CDR’s confer a receptor’s specificity for Ag. 7: In terms of T cells there are two major flavors. Alpha beta T cells express an alpha and beta chain that constitutes the TCR. There is a smaller population called gamma delta because they have a gamma and delta chain for the AgR. In either model you have the variable regions near the amino terminus which are what interact with Ag. This Ag recognition structure on the T cell surface is noncovalently associated with a larger number of polypeptides. On this side you have three polypeptides that form two heterodimers, and this whole complex is known as the CD3 complex, it has a gamma epsilon and an epsilon delta heterodimer. And also the TCR is noncovalently associated with a zeta zeta homodimer, and is important for transducing the signal into the cell once the TCR has recognized the Ag. The blue boxes are ITAMs. It’s a similar phenomenon in that Ag can cause redistribution of the TCR complex in the membrane to localize to bring them closer to the tyrosine kinases which can phosphorylate the ITAMs, and the phosphorylated ITAMs can recruit signaling proteins to the membrane to initiate and propagate signal transduction. 8: T cells are quite different than B cells in the types of Ag they can recognize. B cells can respond to proteins, RNA, DNA, lipids, polysaccharides, or any combo thereof, almost anything in the environment. In contrast, T cells, particularly alpha beta T cells, recognize peptides. That’s it. And those peptides must be presented to the T cell via MHC class II or MHC class I. T cell receptor accessory molecules are CD4 and CD8. 9-10: They are important because when the peptide is presented in the peptide binding groove of MHC class I or II, the T cell receptor will recognize epitopes on the peptide and some aspects of the MHC. The T cell recognizes the peptide-MHC complex. The ability of the T cell to do that is greatly Microbiology Brian Bolerjack Activation of B and T cells 48 minutos 09-03-08 enhanced by the expression of CD4 and CD8. So you have two subclasses, CD4+ and CD8+. T cells expressing CD8 recognize peptides being presented by MHC class I. This is enhanced by CD8’s ability to bind to nonpolymorphic regions in the alpha 3 domain of MHC class I and strengthen the reaction. T cells expressing CD4 recognize peptides being presented by MHC class II. CD4 will bind to nonpolymorphic regions in the beta 2 domain of MHC class II. The accessory molecules play a distinct role in enhancing the reaction of T cells with APCs to receive a sufficiently strong signal to become activated. 11: CD4+ T cells differentiate into Helper T cells which produce cytokines and help regulate the nature of the immune response. CD8+ T cells differentiate into Cytolytic T cells, which will have a role in lysing virally infected cells. Binding of the nonpolymorphic regions by the accessory molecules increases the avidity of the binding between the TCR and the APC and leads to proper signaling and activation. 12: There is another class of receptors on T cells which are also important for activation, and those are adhesion molecules. CD2 (LFA-2) and LFA-1 are adhesion molecules that will bind to counter receptors on the APC, and they help stabilize contact between the TCR and APC. 13: The T cell is up at the top, APC on the bottom presenting peptide on MHC class II. In a lymph node or spleen where T cells and APCs come together, the T cells will be sampling the surface of APCs to determine if it recognizes the peptide. It is initially regulated by for example LFA-1 on the T cell which binds ICAM-1 on the APC with low affinity. It provides just enough contact for the T cell to sample the peptide, and if and when the TCR recognizes a peptide it will drive the signal to the T cell, cause a change in conformation of LFA-1, and LFA-1 will form a high affinity bond with ICAM-1. So you form a contact between the TCR and MHC, the CD4 is also contacting the MHC, and LFA-1 is bound to ICAM-1. The goal of it all is to generate a strong resilient interaction between the T cell and APC so the T cell gets the strength of signal it needs to become fully activated. 14: If you look here at a chart of affinities, there is a large range, but what you will notice is that TCRs are at a very low affinity for peptide MHC, so you must bring in adhesion molecules and other contacts that increase the strength of interaction between the T cell and APC. That is a significant difference between T and B cells in how they perceive and respond to Ag. 15: If and when the cell engages an Ag for which it is specific, that will lead to initiation of signal transduction, changes into gene transcription, entry into the cell cycle, and limited proliferation. If you get no other signals, the T or B cell will undergo apoptosis to ensure that B and T cells only undergo the whole activation, proliferation and differentiation process when they receive the correct cascade of signals that indicate there is indeed a threat to the host. 16: This is just a schematic representation of what happens when a T cell recognizes Ag presented by an APC. You have the TCR that binds to the peptide-MHC complex. In this case you have CD4 or CD8 that will engage the MHC molecule itself. This will initiate redistribution of TCR complexes within the membrane so they will become more localized within the membrane. They migrate into lipid rafts or GEMs- glycolipid enriched microdomains, specialized regions in the plasma membrane. In the rafts you have a higher concentration of SARC family kinases, which are important in phosphorylating the ITAMs in the cytoplasm of CD3 complex or the zeta zeta homodimer. In response to Ag, what you form is called SMAC-supra molecular activation complex. In the complex, the central bullseye is formed by the TCR complexes. Surrounding that is a ring of adhesion molecules, LFA-1. Microbiology Activation of B and T cells 09-03-08 Brian Bolerjack 48 minutos 17-18: That’s critical because it allows the SARC family kinases to phosphorylate the ITAMs within the TCR complex. Step 1: you have redistribution of the TCR into lipid rafts. Step 2: that allows SARC family kinases to phosphorylate the ITAMs to generate phosphotyrosine motifs. Step 3: That recruits another tyrosine kinase, in T cells that’s ZAP-70. It has SH2 domains that bind to these phosphotyrosines. It is recruited and is phosphorylated itself. Step 4: ZAP-70 phosphorylates downstream effectors (adapter proteins and enzymes). Step 5: That leads to activity that alters gene transcription. The whole initiation of activation of T cells is based on tyrosine phosphorylation. 19-20: The B cell is the same paradigm. Ag binding causes B cell receptor complexes in the membrane to localize into lipid rafts. SARC family kinases phosphorylate the ITAMs on Ig alpha/Ig beta. Those recruit the B cell equivalent of ZAP-70 called Syk. Syk phosphorylates downstream adapters and enzymes on tyrosine residues, and they play a major role in driving the major signaling pathways that alter gene transcription. 21: Modulation of BCR-Mediated Signal Transduction. Because B cells can respond to any Ag without presentation, B cells use other types of receptors, co-receptors, to give them some context as to how and when they should respond to the Ag. There should only be one alpha beta heterodimer on the slide. Membrane Ig binds Ag, transduces the signal through Ig alpha, Ig beta, this leads to SARC family kinases phosphorylating the subunits, Syk is recruited and activated, and that phosporylates effectors that mediates signaling and activation. In B cells there are other co-receptors, here there are CD19 and CD22, and their cytoplasmic domains have tyrosine residues and are phosphorylated when Ag binds to the BCR. You are generating phosphotyrosine motifs in the cell which can recruit effector proteins. They can then feed back on the signal pathways regulated by the BCR itself. CD 19 is in green, and when CD 19 is phosphorylated, it recruits proteins that enhance and increase the signal. CD19 is an activating co-receptor. CD22 is in red and when it’s phosphorylated it recruits proteins that attenuate the BCR signal. There are many many co-receptors on B cells. Some enhance activation and others attenuate the BCR signal and diminish B cell activation. Depending on the co-receptor, activation of B cell can be regulated. 22: Skip 23: Costimulation is the last important thing you need to understand. If a T or B cell recognizes Ag through its receptor without another signal through co-stimulatory receptors, it will begin activation, but eventually become anergic, or unresponsive. It can respond to no signals going forward, and will then undergo apoptosis and are eliminated. This protects the body from improper B cell activation from recognizing self Ag. If the system is working well, there won’t be any T cells helping it with costimulation. Over the course of the true Ag process, activated T cells will help the B cells, and give them the costimulatory signal so they can differentiate. If you are infected with a bacteria, you will get activation of APCs, which activate T cells, which can activate B cells to differentiate to respond to the foreign Ag. Microbiology Brian Bolerjack Activation of B and T cells 48 minutos 09-03-08 24: What is the costimulatory molecule required for full T cell activation and differentiation? CD28. All T cells express TCR’s and CD28 constitutively. The T cell gets a signal through the AgR (signal 1) and B7 from APCs bind to CD28 for signal 2, or the co-stimulatory signal. It can bind to B7.1 or CD80 and B7.2 or CD86. That’s the most basic thing you should remember. 25: Signal 2 through CD28 is required to drive optimal proliferation and differentiation of the T cell into its effector cell types. Signals 1 and 2 are important because when they are produced IL-2 receptor expression is upregulated and binding of IL-2 to the receptor is very important in driving proliferation of the clone, which then allows you to generate a large number of effector and memory T cells for the immune response. 26: Skip 27: If you do anything experimentally to block the CD28 signal, you will induce anergy in the T cell if it has encountered Ag alone and it will undergo apoptosis. This picture is from your book and shows you can block the CD28 signal many different ways experimentally. This has been used in therapeutic treatments to shut down the T cell response with autoimmune disease and transplant patients to block transplant rejection. It would be great if you could give a patient an organ and block the CD28 on the T cells that will respond to the organ, and it would effectively prevent rejection. 28: Skip 29: B cell. The important co-stimulatory signal is CD40 which binds to CD40L(igand) (also known as CD154) on the T helper cell. Signal 1 is the Ag being presented by the T helper cell and Signal 2 is CD40 binding CD40L. When T helper cells are activated by APCs they will upregulate expression of CD40L which will allow them to encounter B cells via bound Ag on the MHC class II, and drive the costimulatory signal through the CD40-CD40L interaction. That allows the B cell to undergo full activation. 30: The costimulatory signal from CD40 is important for the majority of the humoral responses during the adaptive immune response. Without the signal, they become anergic and undergo apoptosis. If the B cells get costimulation with the CD40, you get proliferation, clonal expansion, somatic hypermutation which allows the genes encoding the BCR undergo mutation which may enhance their affinity for Ag, class switch recombination which will allow the B cell to change the isotype of Ig it uses which is important depending upon the type of pathogen present. CD40 costimulation promotes differentiation of the B cells into plasma cells that secrete Ab, and memory cells. Without it, you don’t get a majority of the important responses taking place in the humoral response.