Supplementary Information (doc 1084K)
advertisement

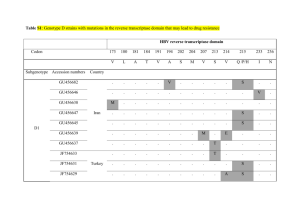
Shahin, Walsh, et al. Supplementary Information: Novel mutations in known deafness genes In 16 of the 20 families evaluated by homozygosity mapping, the longest region of homozygosity included a gene known to be associated with hearing loss (text Table 1). For each of these 16 families, the relevant gene was fully sequenced from genomic DNA of the proband, revealing in 14 families alleles very likely to be deleterious. These alleles included truncating mutations of Otoferlin, TECTA, Pejvakin, and TMPRSS3, a splice site mutation leading to a large in-frame deletion of Pendrin/SLC26A4, and nonsynonymous mutations in TMHS, MYO7A, MYO15A, and CDH23, which were further evaluated by splicing analysis and molecular modeling. Detailed analyses of these point mutations are presented here. OTOF. Family AM was the smallest family analysed by homozygosity mapping (text Figure 1), so not unexpectedly yielded the most deafness-associated homozygous regions. The homozygosity profile revealed five peaks of sizes 2.2, 2.8, 2.9, 3.3, and 19.4 MB (text Figure 2). The only peak spanning a known deafness gene was the largest region at chromosome 2p which includes OTOF, the gene responsible for DFNB9i. Sequence analysis of OTOF in the proband of family AM revealed a novel variant (c4157 C>T) corresponding to a nonsense allele at codon 577. To date, 56 different pathogenic mutations of OTOF have been reportedii,iii. The vast majority of reported alleles are private, described in only one family. The R577X allele was not present in any of the other 218 NSHL probands or in 288 Palestinian hearing controls. In a large study of families with mutations in OTOFiv, 57% of cases with two inactivating alleles had 1 Shahin, Walsh, et al. preserved transient-evoked otoacoustic emissions (TEOAEs), which is consistent with the nonsyndromic hearing loss phenotype of family AM. TECTA. In family BQ, homozygosity mapping yielded a single peak at chromosome 11q23, including TECTA, the gene responsible for dominant hearing loss DFNA8/12 and recessive hearing loss DFNB21. Interestingly, the largest peak of homozygosity in Family BI overlapped the BQ region. Comparison of the SNP haplotypes of families BQ and BI revealed perfect identity across 4MB around the TECTA locus, suggesting a common ancestor, despite ascertainment from different parts of the West Bank and the families being unaware of any relationship. TECTA mutations causing dominant deafness are substitutions of highly conserved amino acid residuesv and an inframe deletion of 37 residuesvi. In contrast, TECTA mutations causing recessive hearing loss are predicted to cause non-functional proteins through truncation or nonsensemediated decayvii,viii,ix. Sequencing genomic DNA from probands of families BQ and BI revealed a TECTA nonsense allele C1619X. This allele was subsequently found to be homozygous in two other Palestinian probands. The audiograms of deaf relatives of these families revealed the ‘U’ shaped curve previously described in DFNB21 families v (Supplementary figure 1). The heterozygous carriers of the allele did not exhibit any hearing loss, an observation consistent with C1619X being a null allele. Pejvakin. Family A had a single homozygous peak spanning Pejvakin (DFNB59). Sequencing revealed a single nucleotide substitution, c762C>T, predicted to alter Arg136 (CGA) to a STOP codon (TGA) in exon 3. To date four premature truncations and six missense alleles of Pejvakin have been described in Iranian, Turkish, and Dutch familiesx’xi,xii. No otoacoustic emission (OAE) distortion products were present in deaf relatives of family A, indicating that auditory neuropathy is highly unlikely to be present. 2 Shahin, Walsh, et al. TMPRSS3. A nonsense allele, TMPRSS3_C194X was identified in family IB, indicated by the largest homozygous region spanning the DFNB8/10 locus. The predicted truncation is within the scavenger receptor cysteine-rich domain, which is N-terminal of the serine protease domain, and hence likely to abolish protease activity. This mutation represents the third different TMPRSS3 truncating allele discovered in Palestinian familiesxiii,xiv whereas missense alleles represent approximately two thirds of the pathogenic alleles in other populationsxv. Pendrin/SLC26A4. Homozygosity mapping of family CB yielded four peaks >2MB, the longest of which includes the gene Pendrin/SLC26A4. Sequencing of SLC26A4 revealed a single base deletion at the last base of exon 11 (c1341G). This mutation has been previously reported in an Arab-Israeli family with Pendred syndromexvi and subsequently in a Turkish family with deafness and goiterxvii, although the consequence of the mutation on SLC26A4 message was not described in these studies. The consequence of SLC26A4.1341G on the SLC26A4 message was evaluated in cDNA of CB1, a hearing parent, and CB3, a deaf child. Total RNA was prepared from whole blood with a Qiagen RNeasy mini kit according to the manufacturer's protocol. Five micrograms of RNA was reversed transcribed with an SLC26A4-specific primer located in exon 14 at nucleotides 1835-1811 (5' TTTGTAATTCTTGGTACTTTTGTAG 3') of the SLC26A4 RefSeq cDNA, accession NM_000411. The resulting cDNA was amplified with a forward primer in exon 8, nt 1232-1251 (5' GCCTCCTGAACTTCCACCTG 3') and a reverse primer in exon 12, nt 1709-1690 (5' ATCCAGCCCCAGAATGATGG 3'). PCR products were electrophoresed on 2% agarose, and splice variants were gel extracted and sequenced in both directions with Big Dye V3.1 on an Applied Biosystems 3130xl instrument. cDNA sequence revealed that SLC26A4.1341G leads to skipping of exon 3 Shahin, Walsh, et al. 11. The predicted effect on the Pendrin protein is deletion of residues 422-447 which encode the tenth transmembrane domain. The deletion of a transmembrane domain is very likely to abrogate transport function. Inner ear computerized tomography (CT) images were performed on individual CB5. The vestibular aqueduct was considered enlarged when the diameter between the common crus and external aperture was 1.5mm or more on thin CT sections. CT analysis of CB5 revealed an EVA of 3.5 mm. Both deaf individuals of family CB were evaluated for goiter. Tri-iodothyronine (T3), tetra-iodothyronine (T4) and thyroid stimulating hormone (TSH) were determined by radioimmunoassay kits. CB3 and CB5 did not exhibit signs of goiter and their thyroid function tests were in the normal range (T3 = 1.3, T4 = 1.6, TSH = 1.15 mlU/ml). TMHS. The longest deafness-associated homozygous region in family DD included the gene encoding TMHS (Tetraspan Membrane protein of Hair Stereocilia). Mutations in TMHS (also known as LHFPL5) are responsible for DFNB66/67xviii,xix. Sequencing TMHS in genomic DNA from the proband of family DD revealed a substitution c1A>G leading to a Met to Val change at codon 1. Translation initiation codon mutations may prevent protein productionxx or translation of a mutant allele initiating from a downstream methioninexxi. The nearest downstream methionine in TMHS is located at residue 28. TMHS is predicted to encode four transmembrane helices with two extracellular loopsxxii. We modeled transmembrane topology of the wildtype protein and the mutant protein initiating from Met 28 using TMpredxxiii. The model strongly favours flipping the orientation of the N terminal domain from intracellular to extracellular in the predicted mutant protein thereby drastically altering function. Four other mutations have 4 Shahin, Walsh, et al. been described in humans with congenital bilateral profound hearing lossxviii,xix and one mutation responsible for the hurry-scurry mousexxii. MYO7A. Homozygosity mapping of family J revealed a 26.4MB peak on chromosome 11. This region contains three known deafness genes: FGF3, responsible for congenital sensorineural deafness with microtia and microdontiaxxiv,xxv; COMT2/LRTOMT, which is responsible for non-syndromic hearing loss DFNB63xxvi,xxvii; and MYO7A, at which recessive alleles cause Usher syndrome type 1Bxxviii and nonsyndromic hearing loss DFNB2xxix,xxx and dominant alleles are associated with DFNA11xxix. Sequence analysis of FGF3 and LRTOMT did not reveal any rare variants. However, in MYO7A, the variant Gly2163Ser segregated with hearing loss. This variant is located in the FERM domain of the myosin tail, and a glycine residue is highly conserved at this site. Gly2163Ser was previously reported in a family with Usher syndrome type 1B as a compound heterozygote in trans with an intronic variantxxxi. Deaf individual Jn17 (age 25) was evaluated by fundoscopy. Visual acuity, color test, and fundus were normal, ruling out retinitis pigmentosa and Usher syndrome. Hearing loss of affected relatives in family J is severe to profound, with thresholds >90dB at all frequencies. The role of MYO7A missense mutations in nonsyndromic hearing loss DFNB2 has been controversial, because homozygosity for all MYO7A truncating mutations syndromexxxii,xxxiii. and most MYO7A missense mutations leads to Usher However, homozygosity for another mutation in the MYO7A tail, Glu1716del, leads to nonsyndromic hearing lossxxxiii. In an assay of the mouse allele homologous to MYO7A_Glu1716del, this mutant protein retained some normal function, localizing along the length of stereocilia in a manner similar to wild-type MYO7A. It is possible that Gly2163Ser leads to a mutant protein that also has some residual activity 5 Shahin, Walsh, et al. enabling normal retinal function, but when co-inherited with a second deleterious allele leads to Usher syndrome MYO15A. Hearing loss in families AN and P mapped to overlapping regions of chromosome 17. Deaf individuals in the two families shared an identical haplotype across a region of 1.54MB around the MYO15A locus (chr17:17,936,632-19,478,062). Sequencing MYO15A in genomic DNA of deaf relatives from both families revealed a novel variant (c7545G>T in exon 35 of NM_016239), segregating with deafness. If it were a missense, this mutation would lead to Asp2403Tyr, in the myosin tail domain between the MyTH4 and FERM domains. However, aspartic acid is not evolutionarily well conserved at this site. MYO15A splicing was evaluated in RNA from whole blood from AN2, a deaf child, using RT-PCR. Total RNA was reversed transcribed with an oligo dT primer and cDNA amplified with primers located GCTGGGACTCGGATGAGGAC-3)' in exon 34, nucleotides 7308-7328 (5'- and exon 36, nucleotides 7662-7643 (5'- GCTTTGGCTCTGGGGGTCTC-3'). Coordinates refer to RefSeq MYO15A cDNA, accession NM_016239. PCR products were sequenced as described for SLC26A4. Sequencing revealed a message splicing from c7544 to the first base of exon 36 (c7551). The c7545G>T mutation led to the creation of an alternate GT donor splice site and deletion of 7 nucleotides (c7544-c7550) from the message. The mis-spliced message alters the reading frame and is predicted to produce 11 novel amino acids prior to the introduction of a nonsense codon. Sequence analysis of the cDNA demonstrated that the nonsense codon is stable and evades nonsense-mediated decay. This allele was homozygous in five of 218 (2.3%) of deaf probands, making MYO15A the third most 6 Shahin, Walsh, et al. common cause of non-syndromic deafness in the West Bank after connexin 26 and TRIOBP. CDH23. Mapping of families AB, DA, and G revealed extended homozygosity in each family on chromosome 10q, in regions including the CDH23 locus. Haplotypes were not shared by deaf relatives from different families, and sequencing revealed three different missense mutations, each homozygous in the deaf individuals in one family: Pro346Ser in family G, Pro346Leu in family DA, and Pro559Ser in family AB. In order to predict changes in the macromolecular structure of CDH23 due to these mutations, we applied molecular modeling tools. A partial CDH23 model was generated by threading the individually aligned CDH23 dimers onto the N terminal domains of the mouse CDH8xxxiv using HHSearchxxxv. Unaligned regions in the CDH23 dimer sequences were rebuilt using fragments from known protein structures and a low-resolution force fieldxxxvi. Positions of calcium ions and calcium-coordinating backbone and side-chain atoms, on the full-sequence model, were inferred by homology to the structure of mouse CDH8. Structures were then refined using the Rosetta full-atom energy function with spatial restraints applied to the starting coordinates in order to prevent inappropriate divergence from the CDH8 structure, following previously published protocolsxxxvii,xxxviii. We used the Rosetta programxxxviii to predict changes in macromolecular structure due to the missense mutations. Cadherin proteins form extended linear structures with interactions only between adjacent domainsxxxiv,xxxix. Every dimer of two cadherin domains contains a full calcium-binding site. Models of CDH23 cadherin dimers constructed using the Rosetta “rebuild and refine” protocol suggest that Pro346 lies between calcium binding domains 3 and 4 and that Pro559 is in the analogous position 7 Shahin, Walsh, et al. between calcium binding domains 5 and 6 (Supplementary figure 2a). All three mutations alter a proline adjacent to the alanine residue whose backbone oxygen is critical to the binding of calcium to cadherin. In the wildtype sequence, these prolines have restricted angular motion and strongly constrain the conformations of the adjacent calcium-binding alanine residues. These angles are predicted to be less strongly constrained in the mutant proteins (Supplementary figure 2b). Structural modeling of calcium binding in cadherin repeats suggests a more general association of amino acid position with functional consequence of missense mutations in CDH23. The published literature on CDH23 includes 22 mutations reported to cause DFNB12, a form of nonsymdromic hearing loss, and 20 nonsynonymous variants reported to be benignxl,xli. The minimum distance from each residue in a cadherin domain to its nearest calcium ion can be predicted with Rosetta tools (Supplementary table 2). Mutations associated with NSHL occur in residues closer to their nearest calcium ion that do mutations reported to be benign: P = 0.009 by 2-tailed Mann-Whitney U-test. 8 Shahin, Walsh, et al. Supplementary table 1. Palestinian families with inherited hearing loss Number of individuals genotyped Nuclear families Deaf Hearing siblings Parents Total A AB AM AN BG BI BQ C CB CG CN DA DD DE DO DP GN IB JN P 2 1 1 1 1 1 2 5 1 4 1 1 2 1 1 2 3 1 1 1 4 4 2 3 3 3 4 11 2 7 3 3 5 3 4 2 5 3 3 4 4 0 1 1 2 0 1 4 1 3 1 0 1 4 1 1 0 0 3 0 4 2 1 2 2 2 4 5 2 6 1 2 3 2 1 1 4 2 2 1 12 6 4 6 7 5 9 20 5 16 5 5 9 9 6 4 9 5 8 5 Total 33 78 28 49 155 Kindred 9 Shahin, Walsh, et al. Supplementary table 2. Variants in cadherin 23 (CDH23) CD1 1 3 3 4 5 5 5 5 6 9 10 10 11 12 13 13 13 14 15 15 15 15 16 16 17 17 18 18 19 19 19 19 20 21 21 22 22 22 23 24 24 25 1 2 AA WT 124 D 346 P 346 P 452 N 480 L 490 G 496 S 559 P 582 R 990 D 1060 R 1061 E 1186 G 1222 A 1341 D 1349 R 1351 D 1437 R 1575 T 1586 A 1595 E 1620 V 1671 T 1675 V 1804 R 1846 D 1887 T 1888 F 1999 T 2044 E 2045 D 2066 R 2148 D 2202 D 2283 V 2358 R 2376 D 2380 P 2465 R 2588 E 2608 R 2635 V V Distance2 G 3.81 L 5.11 S 5.11 S 2.61 Q 13.37 A 6.99 N 10.39 S 5.11 Q 10.90 N 3.01 W 3.58 K 3.76 D 8.88 T 11.56 N 2.33 C 4.25 N 6.69 Q 14.77 A 13.34 P 18.57 K 3.76 M 15.68 S 7.41 I 15.33 Q 17.15 N 3.01 I 7.38 S 7.94 S 10.39 S 13.43 N 11.85 Q 4.22 N 9.66 N 2.33 I 6.07 Q 10.66 N 2.61 L 11.76 W 3.58 Q 13.43 H 3.01 F 14.73 Consequence DFNB12 DFNB12 DFNB12 DFNB12 DFNB12 Benign Benign DFNB12 DFNB12 DFNB12 DFNB12 DFNB12 DFNB12 Benign DFNB12 Benign Benign Benign Benign DFNB12 DFNB12 Benign Benign Benign Benign DFNB12 Benign DFNB12 Benign Benign DFNB12 Benign DFNB12 DFNB12 Benign Benign Benign Benign DFNB12 Benign DFNB12 DFNB12 CD: cadherin domain Predicted distance in angstroms to nearest calcium 10 Shahin, Walsh, et al. Supplementary figure 1. Audiogram of 24 year old with nonsense mutation TECTA.C1619X. 0 10 Left ear Right ear Hearing level (dB) 20 30 40 50 60 70 80 90 100 250 500 1000 2000 Frequency Hz 11 4000 8000 Shahin, Walsh, et al. Supplementary figure 2. Structural modeling of CDH23 mutations. Conserved molecular architecture of cadherin calcium-binding sites is based on crystal structure of mouse CDH8 (PDB ID 2a62). CDH23 cadherin domain (a) Rosetta homology model of the interface of a pair of cadherin domains in human CDH23, including three calcium ions (orange). Between cadherin repeats 3 and 4, mutations at the highly conserved proline346 (yellow star) are predicted to disrupt the conformation of adjacent alanine345 (red star) whose backbone oxygen coordinates binding of a calcium ion. Proline559, which is mutated to serine559 in family AB, is at the analogous site between cadherin repeats 5 and 6. 12 Shahin, Walsh, et al. 0.050 0.045 0.040 0.035 Pro Leu Ser Density 0.030 0.025 0.020 0.015 0.010 0.005 0.000 -150 -100 -50 Phi 0 50 Angle 100 150 200 250 Psi (b) Predicted changes in strength of constraints on conformation of angles at this site given wildtype residue proline vs mutant residues leucine (family DA) or serine (families G and AB). 13 Shahin, Walsh, et al. Genomic regions harboring novel genes for hearing loss in consanguineous families. Supplementary figure 3, on the following pages, illustrates the chromosomal regions linked to loci for hearing loss in families CG, DE, CN, DP, and C, as revealed by homozygosity mapping. (A) DFNB82 at chromosome 1p13.3 in family CG. (B) DFNB83 at chromosome 9p23-p21.1 and 9p13.3-q21.12 in family DE. (C) DFNB84 at chromosome 12q14.3-q21.2 in families CN and DP. (D) DFNB85 at chromosome 17p12-q11.2 in family C. (E) linked region on chromosome 14q23.1-q31.1 in family BG. 14 Shahin, Walsh, et al. (A) DFNB82 1p13.3 Chr1:108,108,849-111,251,931 15 Shahin, Walsh, et al. (B) DFNB83: 9p23-p21.1 and 9p13.3-q21.1; chr9:9,959,023-26,430,090 and chr9:34,401,026-77,904,900 16 Shahin, Walsh, et al. (C) DFNB84 12q14.3-q21.1 chr12:65,434,594-74,498,486 17 Shahin, Walsh, et al. (D) DFNB85 17p12-q11.2 chr17:15,212,798-26,490,848 18 Shahin, Walsh, et al. (E) 14q23.1-q31.1 chr14:57,474,255-80,664,133 19 Shahin, Walsh, et al. References for Supplementary Information i Yasunaga S, Grati M, Cohen-Salmon M et al: A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat Genet 1999; 21: 363-369. ii Choi YE, Butterworth M, Malladi S, Duckett CS, Cohen GM, Bratton SB. The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and -7 via unique mechanisms at distinct steps in their processing. J Biol Chem 2009; 284: 12772-12782. iii Romanos J, Kimura L, Fávero ML et al: Novel OTOF mutations in Brazilian patients with auditory neuropathy. J Hum Genet 2009: 54: 382-385. iv Rodríguez-Ballesteros M, Reynoso R, Olarte M et al: A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy. Hum Mutat 2008; 29: 823831. v Plantinga RF, de Brouwer AP, Huygen PL, Kunst HP, Kremer H, Cremers CW. A novel TECTA mutation in a Dutch DFNA8/12 family confirms genotype-phenotype correlation. J Assoc Res Otolaryngol 2006; 7: 173-181. vi Collin RW, de Heer AM, Oostrik J et al: Mid-frequency DFNA8/12 hearing loss caused by a synonymous TECTA mutation that affects an exonic splice enhancer. Eur J Hum Genet 2008; 16: 1430-1436. vii Mustapha M, Weil D, Chardenoux S et al: An alpha-tectorin gene defect causes a newly identified autosomal recessive form of sensorineural pre-lingual non-syndromic deafness, DFNB21. Hum Mol Genet 1999; 8: 409-412. viii Naz S, Alasti F, Mowjoodi A et al: Distinctive audiometric profile associated with DFNB21 alleles of TECTA. J Med Genet 2003; 40: 360-363. 20 Shahin, Walsh, et al. ix Meyer NC, Alasti F, Nishimura CJ et al: Identification of three novel TECTA mutations in Iranian families with autosomal recessive nonsyndromic hearing impairment at the DFNB21 locus. Am J Med Genet A 2007; 143A: 1623-1629. x Delmaghani S, del Castillo FJ, Michel V et al: Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat Genet 2006; 38: 770-778. xi Collin RW, Kalay E, Oostrik J et al: Involvement of DFNB59 mutations in autosomal recessive nonsyndromic hearing impairment. Hum Mutat 2007; 28: 718-723. xii Hashemzadeh Chaleshtori M, Simpson MA, Farrokhi E et al: Novel mutations in the pejvakin gene are associated with autosomal recessive non-syndromic hearing loss in Iranian families. Clin Genet 2007; 72: 261-263. xiii Scott HS, Kudoh J, Wattenhofer M et al: Insertion of beta-satellite repeats identifies a transmembrane protease causing both congenital and childhood onset autosomal recessive deafness. Nat Genet 2001; 27: 59-63. xiv Walsh T, Abu Rayan A, Abu Sa'ed J et al: Genomic analysis of a heterogeneous Mendelian phenotype: multiple novel alleles for inherited hearing loss in the Palestinian population. Hum Genomics 2006; 2: 203-211. xv Guipponi M, Antonarakis SE, Scott HS. TMPRSS3, a type II transmembrane serine protease mutated in non-syndromic autosomal recessive deafness. Front Biosci 2008; 13: 1557-1567. xvi Everett LA, Glaser B, Beck JC et al: Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 1997; 17: 411-422. 21 Shahin, Walsh, et al. xvii Tekin M, Akçayöz D, Comak E et al: Screening the SLC26A4 gene in probands with deafness and goiter (Pendred syndrome) ascertained from a large group of students of the schools for the deaf in Turkey. Clin Genet 2003; 64: 371-374. xviii Kalay E, Li Y, Uzumcu A, Uyguner O et al: Mutations in the lipoma HMGIC fusion partner-like 5 (LHFPL5) gene cause autosomal recessive nonsyndromic hearing loss. Hum Mutat 2006; 27: 633-9. xix Shabbir MI, Ahmed ZM, Khan SY et al: Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J Med Genet 2006; 43: 634-40. xx Quinteiro C, Castro-Feijoo L, Loidi L et al: Novel mutation involving the translation initiation codon of the growth hormone receptor gene (GHR) in a patient with Laron syndrome. J Pediatr Endocrinol Metab 2002; 15: 1041-5. xxi Nguyen M, He B, Karaplis A. Nuclear forms of parathyroid hormone-related peptide are translated from non-AUG start sites downstream from the initiator methionine. Endocrinology 2001; 142: 694-703. xxii Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zheng QY, Johnson KR. A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci USA 2005; 102: 7894-7899. xxiii Hofmann K, Stoffel W. TMbase - A database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler 1993; 374: 166. xxiv Tekin M, Hişmi BO, Fitoz S et al: Homozygous mutations in fibroblast growth factor 3 are associated with a new form of syndromic deafness characterized by inner ear agenesis, microtia, and microdontia. Am J Hum Genet 2007; 80: 338-344. 22 Shahin, Walsh, et al. xxv Alsmadi O, Meyer BF, Alkuraya F et al: Syndromic congenital sensorineural deafness, microtia and microdontia resulting from a novel homoallelic mutation in fibroblast growth factor 3 (FGF3). Eur J Hum Genet 2009; 17: 14-21 xxvi Du X, Schwander M, Moresco EM et al: A catechol-O-methyltransferase that is essential for auditory function in mice and humans. Proc Natl Acad Sci USA 2008; 105: 14609-14614. xxvii Ahmed ZM, Masmoudi S, Kalay E et al: Mutations of LRTOMT, a fusion gene with alternative reading frames, cause nonsyndromic deafness in humans. Nat Genet 2008; 40: 1335-1340. xxviii Weil D, Blanchard S, Kaplan J et al: Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 1995; 374: 60-61. xxix Liu XZ, Walsh J, Mburu P et al: Mutations in the myosin VIIA gene cause non- syndromic recessive deafness. Nat Genet 1997; 16: 188-190. xxx Weil D, Küssel P, Blanchard S, Lévy G et al: The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat Genet 1997; 16: 191-193. xxxi Janecke AR, Meins M, Sadeghi M et al: Twelve novel myosin VIIA mutations in 34 patients with Usher syndrome type I: confirmation of genetic heterogeneity. Hum Mutat 1999; 13: 133-140. xxxii Astuto LM, Kelley PM, Askew JW et al: Searching for evidence of DFNB2. Am J Med Genet 2002; 109: 291-297. xxxiii Riazuddin S, Nazli S, Ahmed ZM et al: Mutation spectrum of MYO7A and evaluation of a novel nonsyndromic deafness DFNB2 allele with residual function. Hum Mutat 2008; 29: 502-511. 23 Shahin, Walsh, et al. xxxiv Patel SD, Chen CP, Bahna F, Honig B, Shapiro L. Cadherin-mediated cell-cell adhesion: sticking together as a family. Curr Opin Struct Biol 2003; 13: 690-698. xxxv Söding J. Protein homology detection by HMM-HMM comparison. Bioinformatics 2005; 21: 951-960. xxxvi Chu JW, Voth GA. Coarse-grained free energy functions for studying protein conformational changes: a double-well network model. Biophys J 2007; 93: 3860-3871. xxxvii Das R, Qian B, Raman S et al: Structure prediction for CASP7 targets using extensive all-atom refinement with Rosetta@home. Proteins 2007; 69: Suppl 8:118128. xxxviii Ramelot TA, Raman S, Kuzin AP et al: Improving NMR protein structure quality by Rosetta refinement: a molecular replacement study. Proteins 2009; 75: 147-167. xxxix Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E- cadherin rigidification and dimerization. Nature 1996; 380: 360-364. xl Astuto LM, Bork JM, Weston MD et al. CDH23 mutation and phenotype heterogeneity: a profile of 107 diverse families with Usher syndrome and nonsyndromic deafness. Am J Hum Genet 2002; 71: 262–275. xli Roux AF, Faugere V, Le Guedard S et al. Survey of the frequency of USH1 gene mutations in a cohort of Usher patients shows the importance of cadherin 23 and protocadherin 15 genes and establishes a detection rate of above 90%. J Med Genet 2006; 43: 763–768. 24