Accelerated carbonation as a treatment method for MSWI bottom ash
advertisement

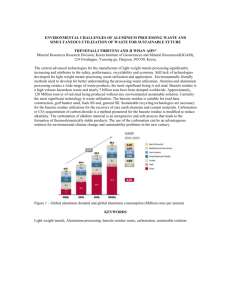
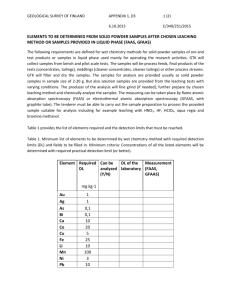
Accelerated carbonation for treatment of MSWI bottom ash S. Arickx, T. Van Gerven, C. Vandecasteele Chemical Engineering Department, Katholieke Universiteit Leuven W. de Croylaan 46, B-3001 Leuven, Belgium Abstract Leaching of MSWI bottom ash exceeds some of the Flemish limit values for heavy metals. In order to recycle bottom ash, treatment is therefore required. The bottom ash studied was divided into four fractions using a large-scale wet sieving installation: sludge ( 0-0.1 mm), sand ( 0.1-2 mm) and two gravel fractions ( 2-6 mm and 6-50 mm). Natural ageing and accelerated carbonation were studied on these fractions. Only the results of the sand fraction are discussed as it is the most interesting fraction because of its non-compliance with the limit values and its potential for recycling. In addition, the velocity of carbonation in a layer of 10 cm sand fraction was investigated. Leaching of Cu, Zn and Pb exceeds the limit value, with Cu – initially exceptionally high – being the most critical. Leaching of Mo, Sb and Ba – only informally regulated in Flanders – is too high as well. Three months of natural ageing was not sufficient for Cu to reach its limit value of 0.5 mg/kg. Moreover, the leaching of Cr increases. Mo and Sb remain above the informal limit value. Four weeks of accelerated carbonation (10% CO 2, 50°C, initial ash humidity of 26%) resulted in a decrease of Cu leaching from 39 mg/kg to 4 mg/kg. However, the limit value is still exceeded. An explanation for the leaching behaviour of the different heavy metals is proposed. To study the velocity of carbonation, the depth of carbonation was measured in a 10 cm thick sample of the sand fraction after different periods of treatment. After three months of natural ageing only the upper 4 cm underwent a significant carbonation, while after one to two weeks of accelerated carbonation the total sample was carbonated. In view of an industrial application, a model was developed to predict the experimental results. Keywords: MSWI bottom ash, recycling, accelerated carbonation, Cu leaching, modelling 1. Introduction Bottom ash is the most important residue originating in the grate furnace of a municipal solid waste incinerator (MSWI) as it accounts for 80-90% of the total weight of all the residues. Although incineration enables to reduce the volume of the waste by up to 90%, the produced amounts of bottom ash are still substantial. In Flanders (the Northern part of Belgium, 5.95 million inhabitants) 3.2 million ton of municipal solid waste was collected in 2003 (VMM, 2004). Roughly 1 million ton was incinerated, resulting in 200,000 to 300,000 ton of bottom ash. Treatment of bottom ash in view of recycling is important in view of sustainable waste management. Bottom ash can replace the sand or gravel fraction in concrete bricks or can be used as such in foundations (IAWG, 1997). For bottom ash to be recycled, it must comply with strict regulations, consisting of civil-technical and environmental requirements. In practice, the latter appear to be a problem as leaching of MSWI bottom ash exceeds some of the limit values for heavy metals, so that treatment is required. Various treatment options applicable to waste incineration residues prior to their reuse or final disposal have been proposed (Sabbas et al., 2003), such as size separation, magnetic separation, washing, ageing/weathering, carbonation, chemical extraction, sintering, … The most commonly used method is natural ageing of bottom ash – which includes reactions as oxidation, carbonation, neutralisation of pH, dissolution, precipitation and sorption – through storage in stockpiles open to the atmosphere for a period of 1 to 3 months (Chimenos et al., 2000). Carbonation has been recognised as one of the most important ageing processes (Kaibouchi and Germain, 2003) and involves the absorption of CO2 by an initially alkaline material, which causes the pH to decrease and calcite to precipitate (Meima et al., 2002). The carbonation reaction can be accelerated using a gas with a higher CO 2 percentage than air. Using the CO2 from the stack gas of MSW incinerators might be an interesting option (Fernández Bertos et al., 2003). To introduce the CO2 needed for carbonation, there are several possibilities: leading the CO2-rich gas in counter-current over a thin layer of bottom ash on a moving belt, or blowing the gas through tubes in the bottom ash heaps (Palitzsch et al., 1999). In this paper, natural ageing and accelerated carbonation are studied on the sand fraction of a bottom ash in order to evaluate their potential for decreasing heavy metal leaching. Accelerated carbonation was also performed using the stack gas of the incinerator. In addition, the velocity of carbonation in a layer of 10 cm sand fraction was investigated. A model for the carbonation reaction was developed and evaluated. 2. Materials and methods 2.1. Materials The MSWI bottom ash used in this study underwent an on-site treatment, consisting of various cut, sieve and wash units in combination with the removal of ferrous and nonferrous fractions. This wet treatment resulted in four fractions: sludge ( 0-0.1 mm), sand ( 0.1-2 mm) and two gravel fractions ( 2-6 mm and 6-50 mm). After collection, the four fractions were dried at 50°C for 9 days and stored in closed buckets to slow down transformation reactions. Although tests were performed on all fractions, only the results of the sand fraction are discussed because of its non-compliance with the Flemish environmental limit values and its high recycling potential. 2.2. Characterisation Leaching of metals from the bottom ash fractions was tested using the EN 12457-2 test. The formally required NEN 7343 column test was also performed on the sand fraction. After filtration of the leachates over a 0.45 µm membrane, the metals in the eluate were measured with a VG PlasmaQuad PQ-2 Plus ICP-mass spectrometer. In order to evaluate the pH dependence of metal leaching from uncarbonated and carbonated (4 weeks) sand fraction, different volumes of concentrated HNO3 or 1M NaOH were added to obtain different leaching pH’s at the end of the EN test. 2.3. Natural ageing experiments Samples of 251 g bottom ash fraction were spread out in several petri-dishes ( 90 mm), which resulted in layers of ca. 3 mm thickness. The petri-dishes were placed in contact with atmospheric air (CO2 percentage of 0.03%) in the laboratory. After 1, 2, 3, 4, 8 and 13 weeks, samples were removed and subsequently tested. To study the depth of carbonation as a function of time, samples of sand fraction were put in several glass recipients of 10 cm height ( 100 mm) and also placed in contact with atmospheric air in the laboratory. After 2, 4, 7 and 12 weeks, a glass recipient was taken and the sample of 10 cm height was divided into 20 subsamples, each consisting of a layer of 0.5 cm. The pH of the leachate was used to evaluate the carbonation rate. 2.4. Accelerated carbonation experiments Accelerated carbonation tests were carried out in a Sanyo MCO-17AI chamber, with the following optimal parameters: 10% CO2, 50°C and an ash humidity of 26%. More details about the experiment and the determination of these optimal parameters are provided in former research (Van Gerven et al., 2005). To verify the possibility of using the stack gas of the incinerator, an experiment was set up at the incineration plant. Stack gas was tapped and pumped through a column ( 48 mm, length 209 mm) packed with sand fraction. After 1, 2, 3 days and 1, 2, 3, 4 weeks the column content was mixed and a sample was taken. Afterwards, the remaining material was loaded back into the column and the removed sample was replaced by inert silica wool. The carbonation rate was studied on samples of sand fraction put in glass recipients of 10 cm height ( 100 mm) in the CO2 chamber. After 1, 2, 3 days and 1, 2, 3, 4 weeks, a glass recipient was taken and tested in the same way as the similar natural ageing test. 2.5. Modelling of carbonation rate Carbonation, the reaction of CO2 and Ca(OH)2 leading to CaCO3 and H2O, is controlled by two mechanisms: CO2 diffusion from the air through the porous system up to the reaction front (at each particle) and the reaction of diffused CO 2 with Ca(OH)2 after dissolution in the thin water film surrounding each particle. These two mechanisms correspond to the first and second term in Eq. (1), the differential equation describing the carbonation process. g CCO 2 t Deff g 2 CCO 2 x2 RCO2 (1) A constant porosity and effective diffusion coefficient Deff are assumed. The reaction term RCO2 is modelled as a second order reaction in CO2 and Ca(OH)2. These concentrations are in the liquid phase and must be converted to the gas phase. The Ca(OH)2 concentration is assumed constant so that the reaction kinetics becomes pseudo-first order and Eq. (1) may be integrated analytically. As the objective of the model is to calculate the pH, a second differential equation Eq. (2) needs to be integrated. From here, pH can be easily calculated as it is controlled by the dissolution of Ca(OH)2. l CCa ( OH ) 2 t RCa (OH ) 2 (2) An iterative scheme is developed to solve the differential system of Eq. (1) - (2) and implemented in Matlab. Several parameters were experimentally measured, e.g. porosity (εair = 0.506, εwater = 0.22). Others were taken from literature, e.g. effective diffusion coefficient (Papadakis et al., 1989). One parameter, the reaction rate constant, was considered as the only degree of freedom and used to fit the experimental results. 3. Results and discussion 3.1. Characterisation of the sand fraction As the metal concentrations obtained with both leaching tests were comparable, the onestage extraction test (EN 12457-2) was further used. As shown in Table 1, limit values for leaching are exceeded for Cu, Zn, Pb, Ba, Mo and Sb (measurements in bold). For the latter three elements however, limit values are not yet officially regulated. The leaching concentrations of Cu, Zn and Pb were exceptionally high. Usually leaching values range from 3-7 mg/kg DM for Cu and from 0.5-3 mg/kg DM for Zn and Pb. Table 1. Leaching concentration [mg/kg DM] of the fresh sand fraction, together with the Flemish limit values for recycling of granular material in construction applications. The values are given as an average of 15 samples together with the standard deviation. As Cd Cu Cr Hg Ni Pb Zn Regulated metals measurement limit value < 0.01 0.8 < 0.01 0.03 0.5 38.8 3.4 0.5 0.16 0.06 n.a. 0.02 0.75 0.17 0.06 1.3 7.6 1.8 2.8 3.8 0.8 Ba Co Mo Sb Se V Non-regulated metals measurement limit value 1.6 7.5 0.5 < 0.01 0.25 0.2 1.1 0.1 0.1 0.5 0.2 < 0.01 0.04 0.8 0.5 0.1 n.a. = not analysed 3.2. Natural ageing The pH of the leachate of samples (sand fraction) subjected to natural ageing decreases gradually from 12.1 to 10.7 after 3 months (Fig. 1). Figure 2 shows the effect of natural ageing on Cu leaching. Three months of natural ageing is clearly not sufficient for Cu to reach its limit value of 0.5 mg/kg DM in these samples. Moreover, the leaching of Cr increases from 0.16 mg/kg DM to 1.1 mg/kg DM. Leaching of Zn and Pb decreases within the first week to below the limit value. Other (informally regulated) metals such as Mo, Sb and Ba still exceed their limit value after three months of ageing. 13 40 Cu leaching [mg/kg DM] pH 12 11 10 9 30 20 10 limit value 0 0 2 4 6 8 10 12 14 0 tim e [w eeks] 2 4 6 8 10 12 14 tim e [w eeks] Figure 1. pH of leachate as a function of time Figure 2. Cu leaching as a function of time The depth of carbonation was measured in a 10 cm thick sample of the sand fraction after different periods of natural ageing. As carbonation is accompanied by a decrease of the pH, the latter parameter can be used to evaluate the extent of carbonation. The results are shown in Figure 3. After three months of natural ageing, only the upper 4 cm underwent significant carbonation. Figure 3. Carbonation test (natural ageing) 3.3. Accelerated carbonation Figure 4 shows the time evolution of the pH of the leachate of samples subjected to accelerated carbonation. Within the first 24 hours an important decrease (form 12.1 to 9.2) occurs, afterwards the pH slowly decreases further to 8.5. This evolution differs from the pH evolution in Figure 1 (natural ageing). The overall influence of accelerated carbonation on Cu leaching (Figure 5) is significant: from 40 mg/kg DM to 4 mg/kg DM. 4 mg/kg DM however still exceeds the limit value of 0.5 mg/kg DM. The main decrease takes place within the first 24 hours. A similar evolution of pH and Cu leaching is already described elswhere (Van Gerven et al., 2004). 40 Cu leaching [mg/kg DM] 13 pH 11 9 30 20 limit value 10 0 7 0 200 400 600 tim e [hours] 800 Figure 4. pH of leachate as a function of time 0 200 400 600 800 tim e [hours] Figure 5. Cu leaching as a function of time To explain the change of Cu leaching from samples exposed to accelerated carbonation for different periods of time (Fig. 5), several parameters have to be considered. As carbonation has a pH effect on the one hand, and a carbonate effect on the other hand, these two parameters were investigated. Figure 6 shows the Cu leaching as a function of pH, both for uncarbonated and carbonated (during 4 weeks) bottom ash (sand fraction). In the pH range from 8 to 12, both pH decrease and carbonate formation seem to be important factors. As calculations with the geochemical speciation program Minteqa cannot explain Cu leaching completely, other factors must be taken in account. Complexation with organic material or sorption to Al-/Fe-oxides are not considered in the model calculations. These factors are currently investigated. uncarbonated 10000 1 0000 1 000 Cu leaching [mg/kg DM] 1 00 carbonated (4 w eeks) 10 1000 1 0 2 4 6 8 1012 14 100 10 1 0 2 4 6 8 pH 10 12 14 Figure 6. pH-variation of Cu For the other regulated metals (Zn, Pb, Ni and Cr), the limit values are complied with within the first hours of accelerated carbonation. For the amphoteric elements Zn and Pb, this is caused by the pH decrease as a consequence of carbonation. Both pH decrease and formation of carbonate cause Ni leaching to decrease. Cr, forming chromate, behaves as oxyanion and has a specific leaching behaviour. Accelerated carbonation was also performed using stack gas from the incinerator, as this gas contains 10% CO2. Despite some problems during the experiment carried out at the incineration plant, caused by condensation of the stack gas on the bottom ash, useful results were obtained. Table 2 shows the evolution of pH and Cu leaching in comparison with the results obtained in the CO2 chamber. The decrease of pH and Cu leaching is slightly faster in the CO2 chamber, but the results appear comparable. This conclusion is also applicable to the leaching of the other regulated metals (not shown). Table 2. Experiment at the incineration plant (use of stack gas) vs. CO2 chamber time [days] 0 1 2 3 7 14 21 28 Incineration plant pH Cu leaching [mg/kg DM] 11.9 36.0 10.5 16.2 10.4 15.8 9.7 13.2 9.6 4.6 9.1 3.8 8.9 5.4 8.6 4.3 CO2 chamber Cu leaching [mg/kg DM] 12.5 38.8 9.2 14.8 8.7 9.9 7.9 8.9 7.9 6.8 8.4 5.1 8.6 5.2 8.6 4.0 pH The carbonation rate in the CO2 chamber was studied by leaching layer by layer (0.5 cm) of a 10 cm thick sample of sand fraction, after different periods of time. After one to two weeks of accelerated carbonation, the total sample was carbonated (Figure 8a). The practical set-up for accelerated waste flue gases carbonation can be achieved in different ways. In Figure 7, one option (leading the stack gas in counter-current over a CO grate layer of bottom ash on a moving belt) is shown. In this process, several carbonation parameters influence the carbonation bottom ash air rate: the thickness of the layer of bottom ash, the velocity of the moving belt (or the residence time of the bottom ash), the CO2 percentage in the air, … For Figure 7. Accelerated carbonation (moving belt) this reason, the previous test (study of the carbonation rate in a 10 cm thick sample of sand fraction) was performed. Modelling of this test would allow variation of the different parameters and calculation of the optimal parameters without having to perform new tests. Figures 8a and 8b show the experimental and modelled results of the accelerated carbonation test. In spite of the many assumptions made, the model enables to predict the trend of pH decrease. This is also the case with the natural ageing test. As the model only takes into account the carbonation reaction, the good correspondence between experimental results and modelling indicates that carbonation is indeed one of the most important reactions in the natural ageing process. However, the simple model cannot predict the experimental results accurately, which implies that also other reactions must play a role in the ageing process. Calculation of optimal parameters is not yet performed in this stage of the development of the model. 2 (a) (b) Figure 8. Experimental results (a) and modelled results (b) of accelerated carbonation test 4. Conclusions In order to recycle the bottom ash sand fraction, treatment is required to lower Cu, Zn and Pb leaching, with Cu – initially exceptionally high (39 mg/kgDM) compared to normal leaching values of 5 mg/kg DM – being the most critical. Leaching of Mo, Sb and Ba is too high as well, but their limit values are to date only informal. Three months of natural ageing is not sufficient for these samples of the sand fraction to comply with the limit values. Cu and Cr leaching still exceed the limit value after this treatment. Mo and Sb remain above the informal limit value. Accelerated carbonation, which can be performed using the stack gas of the incinerator, is a developing technology, which may have potential for the treatment of the bottom ash sand fraction. Cu leaching decreases significantly (from 39 to 4 mg/kg DM) within four weeks of accelerated carbonation, with the main decrease taking place within the first 24 hours, but the limit value is not complied with. However, all other regulated metals besides Cu, do reach the limit value using the accelerated carbonation technique. Possibly a combination of accelerated carbonation with other techniques (e.g. extraction or heating) could result in a Cu leaching to below the limit value. The carbonation reaction was modelled, to study the velocity of carbonation in view of an industrial application. The model shows that carbonation is indeed a major reaction in the ageing process of bottom ash. The model can predict experimental results roughly, but needs further refinement to be applicable. Nomenclature g [ mol / mg3 ] CCO CO2 concentration in the gas phase [m fraction of pores filled with air in the wet bottom ash x [m] depth measured from the upper part of the recipient Deff [ mg3 / mtot .s ] effective diffusion coefficient l CCa ( OH ) 2 [ mol / ml3 ] Ca(OH)2 concentration in the liquid phase 2 3 g 3 tot m ] Acknowledgements S. Arickx acknowledges a scholarship of the FWO-Flanders. The authors are grateful to Indaver N.V. (G. Wauters, M. Jaspers) for the positive support and wish to thank prof. J. Degrève for his help with the modelling. References Chimenos J.M., Fernández A.I., Nadal R., Espiell F., 2000. J. Hazard. Mat. B79, 287. Fernández Bertos M., Johnson D., Simons S., Hills C.D., Carey P., 2003. In: G. Ortiz de Urbina, H. Goumans, (Eds.), WASCON, 4-6 June 2003, San Sebastian, Spain, pp. 913. IAWG, 1997. Municipal solid waste incinerator residues, Elsevier, Amsterdam, The Netherlands. Kaibouchi S. and Germain P., 2003. In: G. Ortiz de Urbina, H. Goumans, (Eds.), WASCON, 4-6 June 2003, San Sebastian, Spain, pp. 645. Meima J.A., van der Weijden R.D., Eighmy T.T., Comans R.N.J., 2002. Appl. Geochem. 17, 1503. Palitzsch S., Marzi T., Beckmann R., Kümmel R., Dohmann J., 1999. Müll und Abfall 3, 129. Papadakis V.G., Vayenas C., Fardis M.N., 1989. AIChE J. 35, 1639. Sabbas T., Polettini A., Pomi R., Astrup T., Hjelmar O., Mostbaue P., Cappai G., Magel G., Salhofer S., Speiser C., Heuss-Assbichler S., Klein R., Lechner P., 2003. Waste Manage. 23, 61. Van Gerven T., K. Imbrechts, E. Van Keer, M. Jaspers, G. Wauters, C. Vandecasteele, 2004. In: Waste Management and the Environment, 29 Sept - 1 Oct 2004, Rhodes, Greece, pp. 3. Van Gerven T., E. Van Keer, S. Arickx, M. Jaspers, G. Wauters, C. Vandecasteele, 2005. Waste Manage. (in press) VMM, 2004. MIRA-T Vlaanderen, D/2004/45/502, LannooCampus, Leuven, Belgium.