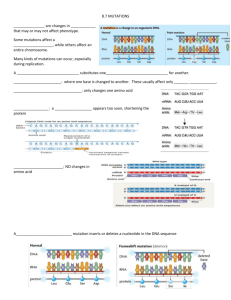
Novel Recurrent Mutations in the Ras-like GTP
advertisement

RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supplementary Appendix NOVEL RECURRENT MUTATIONS IN THE RAS-LIKE GTP-BINDING GENE RIT1 IN MYELOID MALIGNANCIES Inés Gómez-Seguí MD, Hideki Makishima MD, PhD, Andrés Jerez MD, PhD, Kenichi Yoshida MD, BartlomiejPrzychodzen BS, Satoru Miyano, Yuichi Shiraishi, Holleh D. Husseinzadeh, MD, Kathryn Guinta BS, Michael Clemente BS, Naoko Hosono MD, PhD, Michael A. McDevitt MD, PhD, Alison R. Moliterno, MD, PhD, MikkaelA. Sekeres, MD, MS, Seishi Ogawa,MD. PhD, and Jaroslaw P. MaciejewskiMD, PhD. A.Supplementary Methods B. Supplementary Information C. Supplementary Figures and Figure Legends D. Supplementary Tables E. Supplementary Literature Cited 1 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 A. Supplementary Methods Patient population Bone marrow aspirates or peripheral blood samples were collected from 722 patients seen at Cleveland Clinic and Johns Hopkins University School of Medicine (Supp.Table 1). Informed consent for sample collection was obtained according to protocols approved by the individual Institutional Review Boards and in accordance with the Declaration of Helsinki. Diagnosis was confirmed and assigned according to World Health Organization (WHO) classification criteria.1 Lower-risk MDS was defined as patients having <5% myeloblasts. Patients with >5% myeloblasts constituted those with higher-risk disease. To study the germline genotype, immunoselected CD3+ lymphocytes were used. Cytogenetic analysis was performed according to standard banding techniques based on 20 metaphases.Clinical parameters studied included age, gender, hemoglobin, leukocytes, neutrophils, monocytes, platelets, bone marrow blasts, cytogenetics, IPSS risk group2, MDAPS risk group3 or CALGB cytogenetic risk group4 and RIT1 mutation/amplification status. Follow-up of patients was updated on July 2012 and all follow-up data were censored at that point. The median follow-up of surviving patients was 21.3 months (range, 1 to 144 months). Cytogenetics and single nucleotide polymorphism (SNP) array analyses Technical details regarding sample processing for SNP array assays were previously described.5,6 Affymetrix 250K and SNP 6.0 arrays(Affymetrix, Santa Clara, CA) were used. A stringent algorithm was applied for the identification of SNP-A lesions. Patients with SNP array lesions concordant with metaphase cytogenetics or typical lesions known to be recurrent required no further analysis. Changes reported in our internal or publicly-available (Database of Genomic Variants; http://projects.tcag.ca/variation) copy number variation databases were considered non-somatic and excluded. Results were analyzed using CNAG (v3.0)7 or Genotyping Console (Affymetrix). All other lesions were confirmed as somatic or germline by analysis of CD3-sorted cells.8 Whole exome sequencing Whole exome sequencing was performed as previously reported.9 Briefly, tumor DNAs were extracted from patients’ bone marrow or peripheral blood mononuclear cells. For germline controls, DNA was obtained from either paired CD3 positive T cells with or without prior culture in the presence of phytohemagglutinin and IL-2. Whole exome capture was accomplished based on liquid phase hybridization of sonicated genomic DNA having 150 - 200bp of mean length to the bait cRNA library synthesized on magnetic beads (SureSelect®, Agilent Technology), according to the manufacture’s protocol. SureSelect Human All Exon 50Mb kit was used for 20 cases. The captured targets were subjected to massive sequencing using IlluminaGAIIx and/or HiSeq 2000 with the pair end 75-108 bp read option, according to the manufacture’s instruction. The raw sequence data generated from IlluminaGAIIx or HiSeq2000 sequencers were 2 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 processed through the in-house pipeline constructed for whole-exome analysis of paired cancer genomes at the Human Genome Center, Institute of Medical Science, University of Tokyo, which are summarized in a previous report.9 The data processing is divided into two steps, 1) Generation of a .bam file (http://samtools.sourceforge.net/) for paired normal and tumor samples for each case. 2) Detection of somatic point mutations and indels by comparing normal and tumor BAM files. Alignment of sequencing reads on hg19 was visualized using Integrative Genomics Viewer (IGV) software (http://www.broadinstitute.org/igv/).10 Sanger sequencing and allele-specific PCR analysis Exons of selected genes were amplified and underwent direct genomic sequencing by standard techniques on the ABI 3730xl DNA analyzer (Applied Biosystems, Foster City, CA) as previously described.11-13 The allelic presence of T244A, T244G and T245G was also determined by allele-specific PCR.Primers for RIT1 sequencing and RIT1 allele-specific PCR are provided in Supp.Table6 and PCR conditions will be provided if requested. Mutations were detected by bidirectional sequencing and scored as pathogenic if not present in non-clonal paired CD3-derived DNA.RIT1 mutations were named after the NCBI transcript reference sequence NM_006912.5. Coding and sequenced exons to explore concomitant mutations in patients with RIT1 mutations/amplifications are shown in Supp.Table 7. Quantitative RT-PCR by TaqMan probes Total RNA was extractedfrom bone marrow mononuclear cells and cell lines. cDNA was synthesized from 500 ng total RNA using the SuperScript® III First-Strand Synthesis System (Invitrogen). Quantitative gene expression levels were detected using real-time PCR with the ABI PRISM 7500 Fast Sequence Detection System and FAM dye labeled TaqMan MGB probes (Applied Biosystems). TaqMan assays were performed according to the manufacturer’s instructions. The expression level of target genes was normalized to GAPDH mRNA. Primers and probes for all genes analyzed were purchased from Applied Biosystems gene expression assays products (RIT1: Hs00608424_m1; BAD: Hs00188930_m1; BCL2: Hs00608023_m1; MYC: Hs00153408_m1; and GAPDH: Hs99999905_m1). Statistical analysis of clinical data Chi-square and Fisher’s exact tests were used to analyze differences in the distribution of categorical variables among patient subsets. Mann–Whitney–Wilcoxon non-parametric U-test was used to analyze differences in mean ranks. Unadjusted time-to-event analyses were performed using the Kaplan–Meier estimate and for comparisons, log-rank tests. For multivariate analyses, a Cox proportional hazards model was conducted. Variables considered for model inclusion were age, gender, hemoglobin, leukocytes, 3 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 neutrophils, monocytes, platelets, bone marrow blasts, cytogenetics, IPSS risk group, MDAPS risk group or CALGB risk group and RIT1 mutation/amplification status. All computations were performed using the statistical package SPSS, version 17.0 (SPSS Inc., Chicago, IL, USA). A two-sided P value below .05 was considered significant. Publicly available databases and analytical tools The February 2009 human reference sequence (GRCh37) produced by the Genome Reference Consortium was used as reference genome (UCSC genome browser; http://genome.ucsc.edu/cgibin/hgGateway).Expression array data was extracted from Oncomine (https://www.oncomine.org/). Somatic mutation data was searched by Catalogue of somatic mutations in cancer (COSMIC) database in Welcome Trust Sanger Institution website (http://www.sanger.ac.uk/genetics/CGP/cosmic/) and The Cancer Genome Atlas (TCGA) (http://cancergenome.nih.gov/). Each potential mutation was compared against databases of known SNPs, including Entrez Gene (http://www.ncbi.nlm.nih.gov/gene) and the Ensemble Genome Browser (http://useast.ensembl.org/index.html). 4 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 B. Supplementary Information Somatic RIT1 mutations and amplifications in myeloid malignancies We estimated the allelic frequency of the two pilot cases analyzed by NGS to be 35-40%, consistent with 70-80% of the clone size present in heterozygous mutations. Subsequently, we sequenced all 5 coding exons of theRIT1 gene (exons 2-6) in a cohort of 221 patients; 3additional mutant cases were found. All mutations were located in the same aminoacid (F82) in exon 5, other exons were unaffected. These findings prompted us to further expand the screening of mutations in exon 5 to a total of 722 patients with myeloid malignancies, including 200 primary AML cases available for open access data through TCGA(Supplementary Table 1).All together, a total of 9 mutations in 9 patients were found (Supplementary Table 2): in 6 cases the aminoacid F82 was affected (3 F82C, and 1 of each F82I, F82L and F82V), the E81 residue in two cases (E81G and E81Q) and an M90I mutation was found in one patient (TCGA data base). All mutations were located in the Switch II domain of this protein, an effector region very close to the GTP-binding site ‘G3’ that transposes upon activation by GTP and is conserved among species (Fig. 1). All human leukemic cell lines tested showed WT RIT1 (Supp.Table 8). RIT1 belongs to the family of Ras GTPases, which are best known for their ability to serve as molecular switches regulating cell growth, differentiation and survival, mainly through the MEK/ERK and PI3K/AKT pathways.14 Gene mutations that result in expression of constitutively active forms of Ras have been linked to oncogenesis in animal models and humans.15 RIT1 shares high sequence identity (>50%) with the Ras subfamily of GTPases, although it has a highly conserved but distinct G2 effector region and lacks the CAAX motif necessary for plasma membrane association;16 however, Rit is plasma membrane-localized due to a C-terminal cluster of basic amino acids.16 Due to these minor differences from the main Ras family members, Rit can signal both common and unique Ras-responsive elements, even in a cell type-dependent manner, e.g. Rit fails to activate p38-, JNK- or ERK-MAPK or PI3K/Akt pathways in fibroblasts (NIH3T3 cells),17 but is able to stimulate proliferation, differentiation and survival through most of these pathways in neurons (PC6 cells).18-20 Rit1 has also been implicated in promoting stress-dependent cell survival18 and to be critical in the survival of neurons after brain injury.21 Consistent with these results, Rit knockout mice displayed increased apoptosis and selective disruption of MAPK signaling in response to oxidative stress.21,22 and Rit blockade in PC6 shRNAi-treated cells significantly increased apoptosis18 and expression of dominant-negative Rit inhibited neuronal-growth factor-induced neurite outgrowth.19 An experimental form of Rit1 protein carrying the Q79L mutation has been reported to result in neuronal differentiation morphologically different to that caused by oncogeneic Ras23, and inhibits the apoptosis subsequent to growth factor withdrawal in PC6 cells.19 Fibroblasts expressing Rit79L display strong growth transformation and rapid proliferation and even induce tumors in nude mice at sites of injection, showing the tumorigenic phenotype of this activating mutation.17 This mutation, similar (but not identical) to the human somatic mutations found by us in myeloid neoplasms, is also located in the switch II domain adjacent to the GTP-binding site G3, and appears analogous to the Q61 mutation in other Ras family 5 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 proteins such as NRAS or KRAS, but none of the leukemogeneic mutations affected the corresponding residue (Q79) in our cohort. RIT1 has been reported to be both mutated and amplified in hepatocarcinoma. Interestingly, amplification of RIT1 was found in a quarter of HCC cases (11/43) and an additional case (5%) carried the E81G mutation, identical to one of the cases reported in this study.24,25 Other mutations in breast basal carcinoma (n=1), melanoma (n=1) and rectal adenocarcinoma (n=1) can be found in the COSMIC database (Welcome Trust Sanger Institute), although these,unlike the mutations reported in our manuscript, were found in amino acids located toward the N-terminus of the protein (P199P, R112C and D216Y). The canonical nature of RIT1 mutations, coinciding with amplification of the RIT1 locus through whole or partial chromosome duplication in patients with similar phenotype, along with overexpression of putative downstream effectors in patients with Rit abnormalities and its reported transforming capability in in vitro models, suggest that these are activating mutations.In fact, Ras proteins are one of the most common gainof-function mutations found in human cancers26 and novel transforming Ras mutations have been described recently in myeloid cancers outside the known 12,13 and 61 codons, that exhibited oncogenic properties in comparison with wild-type Ras in biochemical and functional assays.27 By the number of affected reads, the RIT1 clone appeares to be dominant; serial testing showed the presence of the RIT1 mutation at initial diagnosis (Suppl. Fig. 1), suggesting that RIT1 may be of ancestral origin. This is supported by the fact that the Nras mutation is sufficient to induce a CMML-like disease in mouse models.28 Parallel analyses using SNP-arrays and sequencing demonstrated that mutations were heterozygous with the exception of one case, which showed a 1q amplification involving the RIT1 locus: in this case the mutant allele was duplicated (Supp. Fig. 2). In 2 cases, the 1q amplification was serially studied: while the post-PV myelofibrosis case showed 1q+ as an acquired event associated with fibrotic transformation, the case of primary myelofibrois displayed it from an early stage (Suppl. Fig. 1), in agreement with a relatively frequent observation of trisomy 1 in primary MF29 and post-PV MF.30 While Ras mutations are rare events in MF and may predict leukemic transformation,31 we did not find RIT1 mutations in classical MPN cases tested. Of note is that the common amplified region of 1q in our cohort was big and involved several candidate genes. RIT1 expression and downtream events The RIT1 gene, like most Ras-related proteins, is ubiquitously expressed,16 including within hematologic tissues, as is shown in publicly available expression array data on lymphocytes (NK, B and T), myeloid cells, monocytes, whole blood and CD34+ progenitors, together with some cell lines of leukemia and lymphoma (MOLT4, K562, DAUDY, HL60) (GeneNote and BioGPS databases). We directly analyzed the expression of the RIT1 gene in cases harboring mutations; cDNA sequencing revealed that mutant allele was expressed equally or even at higher levels than the WT (Suppl Fig. 2). qRT-PCR showed no difference in the expression levels of RIT1 mRNA between mutant and wild-type (WT) cases (median relative ratio of 6 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 0.58 in mutant vs. 0.18 in WT cases; P=0.098), but cases with 1q+ showed significant overexpression (median relative ratio 1.29 vs. 0.18, P=0.041; Fig. 2A). We also explored RIT1 expression in publicly available expression arrays performed in the spectrum of myeloid malignancies (Haferlach leukemia dataset of Oncomine database). The average expression of RIT1 mRNA was increased in AML with complex karyotype and chronic myeloid leukemia (CML) (relative expression 2.2 and 2.3, respectively, vs. 1.8 in healthy donors; P=.016 and P<.001, respectively) (Supp. Table 5). Based on expression of 74 controls, upmodulation of RIT1 (>mean+2 SD of control expression) was found in 9/824 cases (1.1%), remarkably, in 1/38 AML with MLL abnormalities, 3/351 AML with normal karyotype or karyotype with less than two abnormalities, 2/206 MDS and 3/76 CML. RIT1 is a central regulator of a p38 MAPK-dependent signaling cascade that functions as a critical cellular survival mechanism in response to various stress stimuli.18 In particular, RIT1 is able to activate phosphorylation of AKT inhibiting apoptosis, and to activate the classical MAPK cascade regulating proliferation.17,18,22 We therefore studied the expression of putative RIT1 downstream effector genes (i.e.BAD and BCL2, involved in regulation of apoptosis through the AKT pathway, and MYC regulation of proliferation through the classical MAPK pathway) in 6 patients carrying RIT1 mutations and/or amplifications, 7 patients with WT RIT1 as well as healthy donors. Four out of 6 patients (67%) with RIT1 abnormalities showed a lower expression of the BAD gene when compared to healthy donors (median relative BAD expression (median relative expression 0.54vs. 0.98 in healthy donors; P=0.064; although patients with RIT1 WT also had downregulation of the BAD gene (median relative expression 0.43 vs. 0.98 in healthy donors; P= 0.046). Similarly, 3/6 (50%) patients with RIT1 abnormalities had a higher expression of BCL2 (median relative expression 2.88 vs.1.04 for healthy donors; P=0.086). Similarly, patients with RIT1 WT also tended to display upregulation of the BCL2 gene (median relative expression 2.96 vs. 1.04 in healthy donors; P= 0.143). These data were suggestive of a more pronounced AKT pathway activation in most of the patients with RIT1 abnormalities (Suppl.Fig.3B). When MYC gene expression was investigated, patients carrying RIT1 abnormalities showed elevated MYC mRNA levels (median relative MYC expression 2.28 vs. 0.93, in mutants vs. healthy donors, respectively; P=.053), but not patients with WT RIT1 (median relative expression 1.42; P=0.248), suggestive of a stronger proliferative drive in cases with abnormal RIT1 (Suppl.Fig.3B). Molecular and clinical associations of RIT1 mutations and amplifications To clarify the molecular association of RIT1 mutations with other genetic defects, we screened mutations in any of the Ras genes in an expanded cohort of patients studied with myeloid malignancies (n=333) (Supp Table 9 and Supp Table 10). We then expanded the study of concomitant mutations by traditional methods to 7 patients with RIT1 mutations and 4 with 1q amplification (n=10, 1 patient with both mutation and amplification) with available samples for screening of mutations of 17 well-known genes in myeloid cancers (i.e. TET2, IDH1, IDH2, DNMT3A, CBL, RUNX1, ASXL1, UTX (KDM6A), EZH2, NRAS, KRAS, TP53, 7 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 JAK2, SF3B1, U2AF1, SRSF2, and SETBP1). In one atypical RIT1 mutant case with 1q amplification in SNP array we also found a concomitant KRAS mutation, but such a combination was not found in any other patient (Supp. Fig. 1B). The distribution of patients carrying RIT1 mutations or RIT1 amplifications within various malignancies is shown in Fig. 2A. Interestingly, no mutation or amplification was found in lower-grade forms of MDS. Although the diversity of diagnosis and the relatively low frequency of RIT1 abnormalities made it difficult to find discrete clinical correlations, we did discover that RIT1 abnormalities were significantly more frequent in CMML patients than in other patients (56% vs. 9%, respectively; P=0.001) and also were associated with abnormalities in chromosome 7 (33% vs. 6%, respectively; P= 0.017) (Supp. Table 3). When cases with WT RIT1 and those with mutations were compared, mutant and amplified cases showed a trend towards having a shorter median overall survival (19 vs. 14 months, P=0.053). This difference achieved statistical significance when selecting patients with MDS or MDS/MPN diagnosis (24 vs. 16 months, P=0.029; Fig. 2C and Supp. Table 4). However, in multivariate analyses, RIT1 abnormalities lost its association with poor prognosis, which was correlated with older age, higher leukocyte count, hemoglobin level, adverse cytogenetics, or CMML MD Anderson Prognostic Score (MDAPS) (Supp. Table 4A). RIT1 abnormalities did not predict for AML progression. (Supp. Table 4B). Ras mutations are believed to confer a poor prognosis in other solid tumors, but the real impact on outcome is still a matter of controversy in leukemia and may differ between disease subtype; for example, in AML it confers either a poor prognosis, or has no clear prognostic significance in the largest series,32 whereas in JMML it may represent a good-risk marker.33 In our series, patients carrying RIT1 anomalies appeared to have a poor risk, but this may be due to the occurrence of these anomalies in the high-risk subgroups (e.g. patients with chromosome 7 abnormalities). 8 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 C. Supplementary Figures and Figure Legends Supplementary Figure 1(A). Ancestrality of RIT1 mutations and amplifications. Four cases with available sample at different evolutive stages are shown. In UPN 1 and UPN 6 the mutation can be seen at diagnosis, during follow-up and also at the time of AML evolution, all of them apparently in heterozygosis. In UPN15, the log2 ratio of the copy number probes shows gain of 1q at an early stage of myelofibrosis and after years of evolution. In UPN18, 1q amplification cannot be seen in the Polycythemia Vera (PV) stage and appears clearly in the myelofibrotic (MF) phase. (B) Circos diagram showing mutations associated with RIT1 mutants (red) or RIT1 amplifications (orange). One patient carried both a mutation in RIT1 and KRAS and beside, the SNP-A showed RIT1 amplification. 9 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supplementary Figure 2(A). Concomitance of RIT1 amplification and mutation in one patient (UPN4). (B). Mutated allele is equally expressed relative tothe referenced allele, analyzed by parallel genomic DNA and cDNA sequencing. 10 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supplementary Figure 3(A).Relative RIT1 expression in the spectrum of myeloid neoplasms represented in box plots (data analyzed from Oncomine database). Blue bars represent the percentage of each subgroup expressing RIT1 mRNA levels over the median+2SD of the healthy donors. (B) Relative expression of BAD, BCL2 and MYC, all putative downstream effectors of RIT1, in patients with RIT1 activation (by mutations or locus amplifications) and healthy donors. 11 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supp. Table 1. Cohort of patients screened for RIT1 mutations and amplifications. Diagnosis WHO 2008 Myeloproliferative Neoplasms Chronic Myeloid Leukemia RIT1 RIT1 Sequence; N mutations; amplifications; (SNP-A; N) N (%) N (%) 163 (129) 0 (0) 4 (3) 33 (2) 0 (0) 0 (0) Chronic Phase 13 (1) 0 (0) 0 (0) Accelerated Phase 5 (1) 0 (0) 0 (0) Blast Crisis 15 (0) 0 (0) 0 (0) Polycythemia Vera (PV) 34 (34) 0 (0) 0 (0) Post-PV Myelofibrosis 12 (11) 0 (0) 2 (18) Essential Thrombocytemia (ET) 42 (42) 0 (0) 0 (0) Post-ET Myelofibrosis 12 (11) 0 (0) 1 (9) Primary Myelofibrosis 30 (27) 0 (0) 1 (4) 131 (105) 5 (4) 2 (2) Chronic Myelomonocytic Leukemia 72 (50) 5 (7) 2 (4) Juvenile Myelomonocytic Leukemia 37 (36) 0 (0) 0 (0) Refractory Anemia with Ring Sideroblasts with Thrombocytosis 10 (8) 0 (0) 0 (0) AtypicalChronicMyeloidLeukemia 3 (3) 0 (0) 0 (0) Myelodisplastic/MyeloproliferativeNeoplasm, Unclassifiable 9 (8) 0 (0) 0 (0) 119 (79) 1 (1) 2 (3) 5 (4) 0 (0) 0 (0) 13 (11) 0 (0) 0 (0) 2 (1) 0 (0) 0 (0) Refractory Anemia with Ring Sideroblasts 13 (13) 0 (0) 0 (0) Refractory Cytopenia with Mmultilineage Dysplasia 38 (18) 0 (0) 0 (0) Refractory Cytopenia with Excess of Blasts - type 1 22 (13) 0 (0) 1 (8) Refractory Cytopenia with Excess of Blasts - type 2 26 (19) 1 (4) 1 (5) 64 (44) 1 (2) 1 (2) 245 (201) 2 (1) 0 (0) with t(8;21) or inv(16) 24 (18) 0 (0) 0 (0) with t(15;17) 21 (20) 0 (0) 0 (0) with normal karyotype 117 (97) 1 (1) 0 (0) with other abnormalities 55 (41) 0 (0) 0 (0) with complex karyotype 28 (25) 1 (4) 0 (0) 722 (558) 9 (1) 9 (2) Myelodisplastic/MyeloproliferativeNeoplasms Myelodisplastic Syndromes Myelodisplastic Syndrome with isolated del(5q) Refractory Anemia Refractory Cytopenia with Unilineage Dysplasia Secondary Acute Myeloid Leukemia Primary Acute Myeloid Leukemia Total 12 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supp. Table 2. Main characteristics of patients carrying RIT1 mutations and amplifications. UPN Age Gender Diagnosis Karyotype Nucleotide Change Aminoacid change SNP-A gain Chr. Position Size (Mb) Prognosis score 1 67 M CMML-2 46,XY. c.T244A p.F82I - - - 2 79 F CMML1 45,XY,-7. c.T244G p.F82V - - 3 81 M CMML-1 45XY,del(5q),-7. c.T246A p.F82L - - 4 68 M CMML-1 46,XY,+1,der(1;15)(q10;q10). c.A242G p.E81G 1q21.1 - q44 5 56 M CMML-2 46,XY. c.T245G p.F82C - OS Status MDAPS: Int-1 26mo DEAD - MDAPS: Int-2 6mo DEAD - MDAPS: Int-2 5mo DEAD 144083908 - 247185974 103.1 MDAPS: Int-2 AML 16moafter diagnosis 39mo DEAD - - MDAPS: High Refractorydisease 4mo ALIVE AML 35moafter diagnosis 37mo DEAD 2mo DEAD 14mo DEAD 6 70 M RAEB-2 46,XY. c.G241C p.E81Q - - - IPSS:Int-2 7 85 F AML-MRC 46,XX,t(1;3)(p36;q21). c.T245G p.F82C - - - IPSS: High 8 43 F AML- M5B 9 47 F Disease progression Relapse 10moafter diagnosis 46,XX. c.T245G p.F82C - - - CALGB: Int AML- M2 Complex (+8, 7q-, 5q-) C.G270A p.M90I - - - CALGB: Poor 12mo DEAD - - 1p11.1 - q44 121137730 - 247170176 126.03 IPSS: High 6mo DEAD 1mo DEAD 36mo DEAD 32mo DEAD 16mo DEAD 10 63 M AML-MRC 46,XY,+1,der(1;7)(q10;p10), t(5;22)(q13;q11.2). 11 82 F RAEB2 49-53,XX,+X,+1,del(5q),+10,+11,+18,+21,+22. - - trisomy 1 - - IPSS: High 12 59 F CMML-1 46,XX,der(16)t(1;16)(q12;q11.2). - - 1q11.2 - qter 120863833 - 245326460 124.46 MDAPS: Int-2 AML 19 moafter diagnosis 13 65 F CMML-1 47,XX,+9. - - 1q21.1 - q44 143734641 - 247190999 103.46 MDAPS: Int-2 14 58 F RAEB-1 46,XX,del(5q),+9,add(16)(q24). - - 1q21.1 - q44 145231749 - 246948674 101.72 IPSS:Int-2 15 58 M PMF 46,XY,-7,+9,+16,-21,t(1;?). - - 1q21.1 - q44 142756696 - 247110269 104.35 - AML 16 y. after diagnosis 16 y. DEAD 16 51 F Post-PV MF not done - - 1q12 - q44 142215052 - 244658857 102.44 - MF 35 yafterPV diagnosis 4 y. ALIVE - MF 20 y. after ET diagnosis 6 y. ALIVE - MF 22 y. after PV diagnosis 3 y. DEAD 17 18 60 67 M M Post-ET MF Post-PV MF not done not done - - 1q21.1 - q32.1 1q21.2 - q44 142559711 - 202429502 147409484 - 245326460 59.87 97.92 Chr.:chromosome; Mb: megabase; OS: overall survival; mo: months; CMML: chronic myelomonocytic leukemia; RAEB: Refractory Anemia with Excess of Blasts; AML-MRC: Acute Myeloid Leukemia with Myelodisplasia Related Changes; PMF: primary myelofibrosis; MF: myelofibrosis; PV: Policytemia Vera; ET: Essential Trombocytemia; MDAPS: MD Anderson Prognosis Score for CMML; IPSS: International Prognostic Scoring System for MDS; CALGB: The Cancer and Leukemia Group B. 13 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supp. Table 3. Clinical characteristics of myeloid malignancies with or without RIT1 mutations. Age at diagnosis Range Gender (male) Leukocytes (median, x106/L) Hemoglobin (median, g/dL) Platelets (median, x106/L) BoneMarrowBlasts (%) WHO-2008 diagnosis ChronicMyelomonocyticLeukemia Refractory Cytopenia with Excess of Blasts type 2 AML with myelodisplasia related changes primary AML Karyotype normal karyotype complexkaryotype monosomy 7/7qRisk category High risk IPSS (for MDS cohort)(Int-2/high) High risk MDAPS (for CMML cohort)(Int-2/high) High risk CALGB classification (for AML) RIT1 WT n (%) RIT1 mutant (%) 713 (98.8) 61.3 ± 15 18 - 88 207 (40.4) 9 (1.2) 66.2 ± 15 43 - 85 4 (44.4) 5.11 9.6 11.6 9.3 0.360 0.909 76 11 48 9 0.349 0.714 67 (9.4) 25 (3.5) 61 (8.5) 245 (34.3) 5 (55.6) 0.001 1 (11.1) 0.273 1 (11.1) 0.556 2 (22.2) 0.356 241 (48.1) 67 (13.4) 31 (6.2) 4 (44.4) 0.549 1 (11.1) 0.658 0.017 174 (52.9) 40 (60.6) 48 (20.1) 2 (100) 3 (33.3) 5 (100) 1(50) P value 0.342 0.530 0.284 0.094 0.366 14 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supp. Table 4. Survival analyses of patients with RIT1 WT vs. RIT1 with abnormalities (amplifications or mutations). A) Overall Survival Analyses Median OverallSurvival (months) MDS/MDS-MPN/AML patients MDS/MDS-MPN patients High grade MDS (RAEB, AML-MRC) RAEB RAEB-2 AML-MRC CMML primary AML Cox Regression Analysis for Overall Survival High grade MDS (n=94) Cytogenetic Risk (IPSS2) Hemoglobin<10g/dL RAEB (n= 47) Hemoglobin<10g/dL CytogeneticRisk (IPSS2) RAEB2 (n= 24) CytogeneticRisk (IPSS2) AML-MRC (n= 51) CytogeneticRisk (IPSS2) 6 Leukocytecount>20 x10 /L CMML (n= 66) MD Anderson Prognostic Score3 Cytogenetic Risk (Such et al.34) Gender (female) primary AML Age>60 years CytogeneticRisk (CALGB4) RIT1 WT RIT1 mut/amp Pvalue n= 490 19 24 12 15 13 10 17 16 HR n= 14 14 16 6 16 0.5 2.4 26 12 CI 95% 0.053 0.029 0.351 0.612 0.603 0.184 0.594 0.473 P value 1.82 1.06 (1.35 2.45) (1.17 3.63) <0.001 0.012 3.43 1.70 (1.37 8.57) (1.07 2.69) 0.009 0.025 2.48 (1.20 5.12) 0.014 2.08 3.43 (1.37 3.16) (1.47 7.98) 0.001 0.004 1.79 1.58 0.46 (1.25 2.56) (1.02 2.44) (0.22 0.95) 0.003 0.021 0.037 2.82 1.52 (1.92 4.14) (1.14 2.03) <0.001 0.004 15 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 B) Time to AML progression analyses AML progression (%) RAEB CMML Time to 25% of AML progression (months) RAEB CMML Cox Regression Analysis for AML Progression RAEB (n=47) Hemoglobin<10 g/dL CMML (n=66) MD Anderson Prognostic Score RIT1 WT RIT1 mut/amp P value n= 490 n=14 31.8 14.5 33.3 28.6 0.694 0.309 19 29 35 16 0.961 0.511 HR CI 95% P value 4.12 (1.10 - 15.44) 0.036 2.11 (1.07 - 4.12) 0.030 Supp.Table 5.Comparison of median relative mRNA expression of RIT1 in different myeloid malignancies assessed by expression array (Oncomine database). Diagnosis Median Relative RIT1 expression P value* healthydonors (n=74) 1,8494 CBF-AML (n=68) 2,0215 0.732 APL (n=37) AML with NK or other abnormalities (n=351) 1,6323 0.215 1,8331 0.279 AML with MLL abnormalities (n=38) 1,9446 AML with CK (n=48) 2,2081 0.815 0.016 MDS (n=206) 1,8305 0.937 CML (n=76) * compared to healthy donors. 2,3284 <0.001 16 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supp. Table 6. Primers for RIT1 sequencing of coding exons, cDNA of exon 5 and allelespecific PCR for mutant alleles in aminoacid F82. RIT1 Primers for coding exons 5' - 3' sequence Exon2-3_Forward GAGGGACAGGCCAGAATATG Exon2-3_Reverse ACCAACTGCTGATACCCTTG Exon4-5_Forward TTTTAAATAGGCATTTCTTCCG Exon4-5_Reverse CCAAGAATCTGTAAGCCAAGAAAC Exon6_Forward ACTCCAATCTGGGCAACAAG Exon6_Reverse TGAACTTAGTCAAACACACATGG RIT1 Primers for coding exons cDNA_Forward ATCAGCCACCGATTCCCAGAAGAT cDNA_Reverse ATCGTCAGTACGTCGGACTCGATA RIT1 Allele specific PCR (T244A) RIT1_T244A_FI ATGACCCTTGTTTCCCTCTAGGCAGTGA RIT1_T244A_RI TCATATACTGGTCCCGCATGGCTGTTAA RIT1_T244A_FO TGGACATTTTGGATACAGCTGGACAGGT RIT1_T244A_RO TGACTTGTTTCCCACAAGAACCACAGGT RIT1 Allele specific PCR (T244G) RIT1_T244G_FI ATGACCCTTGTTTCCCTCTAGGCAGGGT RIT1_T244G_RI CATATACTGGTCCCGCATGGCTGTGAC RIT1_T244G_FO GGCTTTTTTACTCTAGCAAAGGGGAGGGG RIT1_T244G_RO TCCCACAAGAACCACAGGTGTATCGTCA RIT1 Allele specific PCR (T245G) RIT1_T245G_FI TGACCCTTGTTTCCCTCTAGGCAGAATG RIT1_T245G_RI CATATACTGGTCCCGCATGGCTGCAA RIT1_T245G_FO GGCTTTTTTACTCTAGCAAAGGGGAGGGG RIT1_T245G_RO TCCCACAAGAACCACAGGTGTATCGTCA 17 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supp. Table 7. List of genes and their annotation screened by Sanger sequencing or ARMS-PCR Amino Coding Sequenced Genes EnsemblecDNAsequence acids exons exons TET2 ENST00000380013 2002 3-11 3-11 IDH1 ENST00000345146 414 3-10 4 IDH2 ENST00000330062 452 2-11 4 DNMT3A ENST00000264709 912 2-23 21-23 CBL ENST00000264033 906 1-16 8, 9 RUNX1 ENST00000300305 480 2-9 2-9 ASXL1 ENST00000375687 1541 1-13 13 KDM6A (UTX) ENST00000377967 1401 1-29 1-29 EZH2 ENST00000320356 751 2-20 2-20 NRAS ENST00000369535 189 2-5 2 KRAS ENST00000311936 188 2-5 2 JAK2 ENST00000381652 1132 2-24 13* TP53 ENST00000269305 393 2-11 5-8 SETBP1 ENST00000282030 1596 2-6 2-6 SF3B1 ENST00000335508 1304 1-25 14-15 U2AF1 ENST00000291552 240 1-8 2, 6 221 1-2 1 SRSF2 ENST00000392485 * JAK2 V617F was screened by ARMS-PCR. Supp. Table 8. Mutational status of RIT1 gene in 17 human leukaemia cell lines Cell line CMK-2 GDM-1 HEL HL60 KASUMI KG-1 KMS-12 KU-812 MEG-01 MEG-A2 MOLM-13 MUTZ-8 NKM-1 SKM-1 TF-1 THP-1 UT-7 RIT1exon 4-5 mutational status WT WT WT WT WT WT WT WT WT WT WT WT WT WT WT WT WT 18 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supp. Table 9. Diagnosis distribution of the cohort studied by whole exome sequencing. Diagnosis Total AML (n=200) lowrisk 38 Intermediate 127 high-risk 35 MDS (n=65) low grade 44 25 high grade MDS/MPN (n=36) 24 CMML 12 other MDS/MPN secondary AML (n=32) TOTAL 333 Therapy-related condition (n=19) 19 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 Supp. Table 10. Mutations found in patients studied by whole exome sequencing and carrying Ras family mutations showed in Figure 2D. Gene Patient 1 (sAML) NRAS BCOR DNMT3A IDH1 STAG2 Patient 2 (MDS) NRAS NRAS ASXL1 BCOR DNMT3A RUNX1 Patient 3 (AML) NRAS DNMT3A FLT3 IDH1 NPM1 Patient 4 (AML) NRAS DNMT3A NPM1 Patient 5 (AML) NRAS DNMT3A ETV6 IDH2 Patient 6 (AML) NRAS DNMT3A NPM1 CEBPA PTPN11 WT1 Patient 7 (AML) NRAS DNMT3A EZH2 IDH1 Patient 8 (AML) NRAS DNMT3A NPM1 NM number Genome Position Mutation NM_002524 NM_001123383 NM_153759 NM_005896 NM_001042751 115258747 39934196 25462009 209113113 123220476 p.G12D p.S135fs p.G611S p.R132S p.R1045X NM_002524 NM_002524 NM_015338 NM_017745 NM_175629 NM_001001890 115258744 115258748 31023070 39922019 25467449 36171602 G13D G12S S852X V1351fs G543C L294fs NM_002524 NM_022552 NM_004119 NM_005896 NM_002520 115258744 25457243 28592642 209113112 170837547 p.G13D p.R882C p.D835Y p.R132H p.W288fs NM_002524 NM_022552 NM_002520 115258744 25468163 170837547 p.G13D p.E505* p.W288fs NM_002524 NM_022552 NM_001987 NM_002168 115258744 25463163 12043874 90631934 p.G13V splice_site splice_site p.R140Q NM_002524 NM_022552 NM_002520 NM_004364 NM_002834 NM_024426 115258744 25468133 170837547 33792294 112939981 32417907 p.G13D p.Q515* p.W288fs p.R343fs p.I545L p.A382fs NM_002524 NM_022552 NM_004456 NM_005896 115258747 25463529 148506443 209113113 p.G12D p.P718L p.R690H p.R132C NM_002524 NM_022552 NM_002520 115258748 25463286 170837547 p.G12C p.R736H p.W288fs 20 RIT1 mutations in myeloid neoplasia Gene NM number Patient 9 (AML) NRAS NM_002524 DNMT3A NM_022552 DNMT3A NM_022552 U2AF1 NM_001025203 Patient 10 (AML) NRAS NM_002524 TET2 NM_017628 TET2 NM_017628 U2AF1 NM_001025203 Patient 11 (AML) NRAS NM_002524 RUNX1 NM_001754 STAG2 NM_001042749 NM_017628 TET2 NM_017628 TET2 Patient 12 (AML) NRAS NM_002524 NPM1 NM_002520 IDH1 NM_005896 Patient 13 (AML) NRAS NM_002524 Patient 14 (AML) NRAS NM_002524 Patient 15 (MDS) NRAS NM_002524 PDGFRA NM_006206 Patient 16 (JMML) NRAS NM_002524 Patient 17 (sAML) NRAS NM_002524 ZRSR2 NM_005089 Patient 18 (AML) NRAS NM_002524 CSF3R NM_156039 Patient 19 (AML) NRAS NM_002524 CUL1 NM_003592 Patient 20 (MDS) NRAS NM_002524 Patient 21 (AML) NRAS NM_002524 Patient 22 (AML) NRAS NM_002524 Patient 23 (AML) NRAS NM_002524 CEBPA NM_004364 Patient 24 (AML) NRAS NM_002524 TP53 NM_000546 Gómez-Seguí et al. 2013 Genome Position Mutation 115256528 25463271 25469029 44524456 p.Q61H p.A741V p.E477* p.S34F 115256529 106157969 106156747 44524456 p.Q61R p.Q958fs p.R550* p.S34F 115256529 36231783 123220476 106196213 106183007 p.Q61P p.R201* p.R1045* p.R318* splice-site 115258747 170837547 209113113 p.G12D p.W288fs p.R132C 115258748 p.G12C 115256529 p.Q61L 115258747 55129915 p.G12D p.C150F 115258748 p.G12C 115258748 15836719 p.G12S p.N261Y 115258744 36933434 p.G13D p.T618I 115256528 148454181 p.Q61H p.N141S 115258744 p.G13D 115256530 p.Q61K 115256530 p.Q61K 115258747 33792395 p.G12D p.309in_frame_insV 115258747 7577081 p.G12D p.E286G 21 RIT1 mutations in myeloid neoplasia Gene NM number Patient 25 (AML) NRAS NM_002524 NPM1 NM_002520 Patient 26 (AML) KRAS NM_033360 DNMT3A NM_022552 NPM1 NM_002520 U2AF1 NM_001025203 Patient 27 (AML) KRAS NM_033360 DNMT3A NM_022552 IDH2 NM_002168 Patient 28 (AML) KRAS NM_033360 DNMT3A NM_022552 NPM1 NM_002520 PDGFRA NM_006206 Patient 29 (AML) KRAS NM_033360 DNMT3A NM_022552 IDH2 NM_002168 ASXL1 NM_015338 Patient 30 (CMML) KRAS NM_033360 ATM NM_000051 SRSF2 NM_003016 TET2 NM_017628 Patient 31 (CMML) KRAS NM_033360 CSF3R NM_000760 NF1 NM_000267 TET2 NM_001127208 TET2 NM_001127208 Patient 32 (CMML) KRAS NM_033360 ASXL1 NM_015338 CBL NM_005188 EZH2 NM_004456 LUC7L2 NM_016019 U2AF35 NM_006758 Patient 33 (sAML) KRAS NM_033360 Patient 34 (AML) KRAS NM_033360 SRSF2 NM_003016 Patient 35 (AML) KRAS NM_033360 Patient 36 (AML) KRAS NM_033360 Gómez-Seguí et al. 2013 Genome Position Mutation 115258744 170837547 p.G13D p.W288fs 25398284 25467032 170837547 44524456 p.G12V p.Q615* p.W288fs p.S34Y 25378562 25467106 90631934 p.A146T p.G590fs p.R140Q 25398281 25457242 170837547 55156567 p.G13D p.R882H p.W288fs p.G990R 25380282 25457242 90631838 31023271 p.A59E p.R882H p.R172K p.S921fs 25378562 108155188 74732959 106157684 p.A146T p.L1327fs p.P95L p.L862fs 25398262 36932224 29684326 106164897 106156747 p.L19F p.Q749X p.R?* p.Y1255X p.R550X 25398306 31022592 119149003 148506205 139060903 44514777 p.K5E p.R693X p.W408L p.K718fs splice-site p.Q157R 25398284 p.G12D 25398284 74732389 p.G12V p.173in_frame_insKS 25398281 p.G13D 25398284 p.G12V 22 RIT1 mutations in myeloid neoplasia Gene NM number Patient 37 (AML) KRAS NM_033360 SETBP1 NM_015559 Patient 38 (AML) KRAS NM_033360 SF3B1 NM_012433 Patient 39 (CMML) RIT1 NM_006912 RUNX1 NM_001122607 SF3B1 NM_012433 STAG2 NM_001042751 STAG2 NM_001042751 TET2 NM_017628 Patient 40 (CMML) RIT1 NM_006912 Patient 41 (MDS) PRPF8 NM_006445 RIT1 NM_006912 STAG2 NM_006603 Patient 42 (AML) EGFR NM_005228 RIT1 NM_006912 TP53 NM_000546 TP53 NM_000546 Gómez-Seguí et al. 2013 Genome Position Mutation 25398211 42531907 p.I36M p.D814N 25398284 198266834 p.G12D p.K700E 155874287 36252940 198267491 123197737 123197737 106158302 p.F82I p.S114X p.E622D p.E621fs p.E621K p.Q1068fs 155874285 p.F82L 1565301 155874286 123224540 p.M1307I p.F82C p.R1131fs 55238900 155874261 7578414 7578206 p.T638M p.M90I p.V173fs p.S215G 23 RIT1 mutations in myeloid neoplasia Gómez-Seguí et al. 2013 E. Supplementary Literature Cited 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. Swerdlow, S. et al.WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues., (IARC press, Lyon, France, 2008). Greenberg, P. et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89, 2079-2088 (1997). Onida, F. et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood 99, 840-9 (2002). Byrd, J. et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 100, 4325-36 (2002). Maciejewski, J.P., Tiu, R.V. & O'Keefe, C. Application of array-based whole genome scanning technologies as a cytogenetic tool in haematological malignancies. Br J Haematol 146, 479-88 (2009). Gondek, L.P. et al. Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood 111, 1534-42 (2008). Nannya, Y. et al. A robust algorithm for copy number detection using high-density oligonucleotide single nucleotide polymorphism genotyping arrays. Cancer Res 65, 6071-9 (2005). Tiu, R.V. et al. New lesions detected by single nucleotide polymorphism array-based chromosomal analysis have important clinical impact in acute myeloid leukemia. J Clin Oncol 27, 5219-26 (2009). Yoshida, K. et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478, 64-9 (2011). Robinson, J.T. et al. Integrative genomics viewer. Nat Biotechnol 29, 24-6 (2011). Dunbar, A.J. et al. 250K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res 68, 10349-57 (2008). Jankowska, A.M. et al. Loss of heterozygosity 4q24 and TET2 mutations associated with myelodysplastic/myeloproliferative neoplasms. Blood 113, 6403-10 (2009). Makishima, H. et al. CBL, CBLB, TET2, ASXL1, and IDH1/2 mutations and additional chromosomal aberrations constitute molecular events in chronic myelogenous leukemia. Blood 117, e198-206 (2011). Takai, Y., Sasaki, T. & Matozaki, T. Small GTPbinding proteins. Physiol Rev 81, 153–208 (2001). Conti, C. Mutations of genes of the ras family in human and experimental tumors. Prog Clin Biol Res 376, 357-78 (1992). Lee, C.-H.J., Della, N.G., Chew, C.E. & Zack, D.J. Rin, a Neuron-Specific and Calmodulin-Binding Small G-Protein, and Rit Define a Novel Subfamily of Ras Proteins. The Journal of Neuroscience 16, 6784-6794 (1996). Rusyn, E.V. et al. Rit, a non-lipid-modified Ras-related protein, transforms NIH3T3 cells without activating the ERK, JNK, p38 MAPK or PI3K/Akt pathways. Oncogene 19, 4685-94 (2000). Shi, G.X., Jin, L. & Andres, D.A. A rit GTPase-p38 mitogen-activated protein kinase survival pathway confers resistance to cellular stress. Mol Cell Biol 31, 1938-48 (2011). Spencer, M.L., Shao, H. & Andres, D.A. Induction of Neurite Extension and Survival in Pheochromocytoma Cells by the Rit GTPase. Journal of Biological Chemistry 277, 20160-20168 (2002). Andres, D.A., Rudolph, J.L., Sengoku, T. & Shi, G.X. Analysis of Rit signaling and biological activity. Methods Enzymol 407, 499-512 (2006). Cai, W. et al. Rit GTPase signaling promotes immature hippocampal neuronal survival. J Neurosci 32, 9887-97 (2012). Cai, W. et al. An evolutionarily conserved Rit GTPase-p38 MAPK signaling pathway mediates oxidative stress resistance. Mol Biol Cell 22, 3231-41 (2011). 24 RIT1 mutations in myeloid neoplasia 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. Gómez-Seguí et al. 2013 Hynds, D.L., Spencer, M.L., Andres, D.A. & Snow, D.M. Rit promotes MEK-independent neurite branching in human neuroblastoma cells. J Cell Sci 116, 1925-35 (2003). Li, J.T. et al. Amplification of RIT1 in hepatocellular carcinoma and its clinical significance. Ai Zheng 22, 695-9 (2003). Li, J.T. et al. Mutation and amplification of RIT1 gene in hepatocellular carcinoma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 21, 43-6 (2004). Bos, J. Ras oncogene in human cancer: a review. Cancer Res 49, 4682-9 (1989). Tyner, J.W. et al. High-throughput sequencing screen reveals novel, transforming RAS mutations in myeloid leukemia patients. Blood 113, 1749-1755 (2009). Wang, J. et al. Endogenous oncogenic Nras mutation initiates hematopoietic malignancies in a doseand cell type-dependent manner. Blood 118, 368-379 (2011). Reilly, J., Wilson, G., Barnett, D., Watmore, A. & Potter, A. Karyotypic and ras gene mutational analysis in idiopathic myelofibrosis. Br J Haematol 88, 575-81 (1994). Andrieux, J. et al. Karyotypic abnormalities in myelofibrosis following polycythemia vera. Cancer Genet Cytogenet 140, 118-23 (2003). Reilly, J.T. Cytogenetic and Molecular Genetic Abnormalities in Agnogenic Myeloid Metaplasia. Seminars in oncology 32, 359-364 (2005). Kadia, T.M. et al. Clinical and proteomic characterization of acute myeloid leukemia with mutated RAS. Cancer 118, 5550-5559 (2012). Yoshida, N., Doisaki, S. & Kojima, S. Current management of juvenile myelomonocytic leukemia and the impact of RAS mutations. Paediatr Drugs 14, 157-63 (2012). Such, E. et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica 96, 375-83 (2011). 25