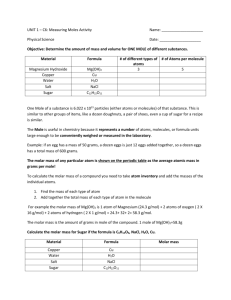
Enhancement Ideas, Chapter 2: An Overview of Materials and
advertisement

Enhancement Ideas, Chapter 2: An Overview of Materials and Reactions
Question 2-1: Skill development, Dimensional Analysis
Any time you have to do mathematical manipulations as a means of solving a chemistry problem, you
can verify your answer through the application of dimensional analysis. Dimensional analysis is
simply the act of using “units” to either;
a)
b)
Arrive at a formula with which to do your calculations or
Use an existing formula and verify that you have the correct answer by cancelling units
In the first case, I will use the units of molecular weight to derive a formula. Molecular weight
has units of grams per mole. That is Mw=grams/moles. Grams are a unit of mass and mole
amount is represented by the letter n. Therefore Mw = m/n.
Here’s another example;
We’ll use an existing formula and dimensional analysis to verify a calculation.
From physics we have the formula; Kinetic Energy = Ke = ½ mv2. Where m is mass, and v is
velocity. Mass must be in kg and velocity must be in metres per second.
Expected and accepted, units of energy are kg.m2s-2. {s-2 means the same as; 1/s2}
Given a baseball with a mass of 0.15 kg and a velocity of 52 metres/second, what is its Kinetic
energy?
Solution; Ke = ½ mv2 = ½(0.15 kg)(52 m/s)2 = 202.8 kg.m2/s2. Notice our derived units are the
units we would expect if our calculation was correctly performed. If instead of using kg we had
used 150 g for our mass, our answer would be in g.m2/s2 and not kg.m2/s2. The answer would be
wrong and this would be clear to us because the expected answer has units that do not match.
Note: It is advisable, and generally required by most instructors, that you always include your
units in your calculations as a self-check tool of dimensional analysis as well as for clarity.
1.
i. Calculate the number of seconds in 15 days
ii. Derive the formula for the universal gas constant, R, if its units are; L.atm/mole.K {K stands for
Kelvin, a unit of temperature}
iii. What is the formula for velocity if its units are metres/second
iv. Convert the value 35.6 m/s to units of ft/hr. (1 foot = 0.3048m)
ANS:
i.
Number of seconds in 15 days. Use unitary rates to solve this problem. A unitary rate is
either a fraction where some value is over the number 1 or the inverse, where 1 is over
Copyright © 2011 Nelson Education Limited
2-1
some value.
To create unitary rates, use known relationships. You know that there are 60 seconds in 1
minute, therefore; 1 min/60 s or 60 s/1 min. Both are correct and each is a unitary rate.
You also know 60 min/1 hr and 1hr/60 min. Lastly, you know there are 24 hr/day and 1
day/24 hr.
Now arrange them sequentially such that the units that you want are left intact, and units
that you don’t want are cancelled. Start with your given value and multiply. If you arrange
your unitary rates incorrectly (invert inappropriately), your units won’t cancel and this will
tell you that your answer is wrong.
Ie.
15 days x (24 hours/1day) x (60 min/hr) x (60 s/min) = 1,296,000 s
Check! We expected units of seconds and arrived at units of seconds, therefore
our answer must be correct.
ii.
Formula for the universal gas constant, R, given R has units of L.atm/mole.
K L is a unit of volume, V. And atm is short for atmosphere. It is a unit of pressure, P. Mole
has the symbol, n, and K is a unit of temperature, T.
Therefore, R = V.P/n.T
You may recognize this as a rearrangement of the universal gas law equation PV = nRT.
iii.
Units of velocity are m/s. Therefore the formula for velocity, v=(distance/time) = d/t
iv.
35.6 m/s to ft/hr.
(35.6 m/s) x (60s/hr) x (1ft/0.3048m) = 7007.87 ft/hr = 7010 ft/hr
to the correct number of significant digits.
REF: Appendix G
Question 2-2: Skill development, General Problem Solving Strategies
Many questions on chemistry exams and quizzes are presented as narrative problems. There are
some strategies you can use to simplify the solving of the majority of such questions. The general
methodology is as follows;
i.
List what is given to you in the problem
ii.
State what concept or value is expected as a solution to the problem
iii.
Search your memory for a formula that can be used that includes the variables given (the
values) and the variable that is required, the unknown. If there is no formula that you can
recall then derive an equation using the units of the expected answer and the tools of
dimensional analysis (see prior skill).
Copyright © 2011 Nelson Education Limited
2-2
iv.
Arrange the equation to solve for your target answer (unknown variable).
For example;
A graduated cylinder containing 25.00 mL of water with a combined mass of 57.45 grams has
approximately 3.00 tablespoons of sand added to it. The volume after addition of the sand is 37.5
mL. The mass of the cylinder, water, and sand, after addition is 67.25 g. What is the mass and
density of the sand?
Apply the methodology;
Given;
Water mass + cylinder mass = 57.45 grams
Water mass + cylinder mass + sand mass = 67.25 g
Volume of water alone = 25.00 mL
Volume of water + volume of sand = 37.5 mL
Approximate volume sand = 3 tbsp
Wanted;
Density of sand
Mass of sand
Ask yourself, are there any formulas I know that include these values, mass and volume.
Answer, yes. Density includes mass and volume. Density = mass/unit volume
Therefore, density of sand = mass sand/volume sand
Were we given either of these values? Yes, we were given volume, but very imprecisely. Can we
calculate precise values with the info given? Yes we can.
Calculate the mass of the sand from change of mass. It is indirectly given as the change in mass upon
addition of sand to the cylinder.
msand + mwater + mcylinder
67.25 g
mwater + mcylinder
-57.45 g
= 9.80 g of sand
What about the volume of sand? Isn’t it given. Yes, it is but the volume is only approximate and
can’t be used. We want a more precise volume. It can be calculated from the change in volume
upon addition of the sand (the displacement).
Volume of sand = (volume of sand + water) – volume of water alone.
= 37.5 mL – 25.00 mL = 12.5 mL
Now we have all of the info we need to calculate density of the sand. Density = m/v
Density of sand = mass of sand/volume of sand = 9.80g/12.5 mL = 0.784 g/mL
Copyright © 2011 Nelson Education Limited
2-3
However, density of solids is typically in g/cm3 and 1 cm3 = 1 mL , therefore the density of the sand is
0.784 g/cm3
2. a) Do question 2.44 from page 39 of your text.
b) Do question 2.46 from page 39 of your text.
ANS:
a) ratio of number of molecules in 100 moles of nitrogen gas (N2) to the # of molecules in 300
mol of H2.
Given:
100 moles of nitrogen gas
For every mole of N2 there is one mole of N2 molecules, by definition
300 moles of H2
For every mole of H2 there is one mole of H2 molecules, by definition
Wanted:
Ratio of molecules N2 to molecules of H2
Solution;
Since molecules N2 = moles N2 and molecules H2 = moles H2 then;
Molecules N2/molecules H2 = moles N2/moles H2 = 100 moles N2/300 moles of H2 = 0.3
b) Mass of zinc that has same number of atoms as 6.355 g of copper.
Given;
Mass of copper = 6.355 g
From periodic table, Mw of Cu = 63.546 g/mole
6.022 x 10 23 atoms/ mole of atoms, by definition of the mole
Wanted;
Mass of zinc with same number of atoms as given mass of copper
Solution;
This problem asks for a mass of zinc but we are given only a mass of copper. How can we get
from mass to # of atoms? Do we have a formula? No, we don’t. But we can calculate moles of
atoms using the formula for atomic weight. Mw = m/n. Then, if we understand that a mole is
simply a number then we can convert moles of copper to actual particles; in this case atoms.
Ie,
n= m/Mw = 6.355 g/(63.546 g/mole) = 0.100 moles
then;
0.100 moles Cu x 6.022 x 10 23 Cu atoms/ mole Cu = 6.022 x 10 22 Cu atoms
Now, relate to the question. We want the mass of zinc with the same number of atoms. If we
new the mass of a single atom of zinc we could simply multiply atoms by mass/atom to arrive at
Copyright © 2011 Nelson Education Limited
2-4
total mass. Do we have a formula with mass and # of atoms. No, but, once again we do have the
equation for Mw = m/n. And, n = 1 mole when stated as an atomic weight. That is zinc has an
atomic weight of 65.38 g/1 mole. And we know that 1 mole = 6.022 x 10 23 atoms. Thus,
(65.38 g Zn/mole Zn) x [1 mole Zn/(6.022 x 10 23 atoms Zn)] = 1.0857 g Zn/atom Zn
Now,
1.0857 g /atom x 6.022 x 10 22 atoms = 6.538 grams
Note: there was a simpler solution to this problem that simply relates moles to moles. Can you
solve it?
REF: Section 2.11 of text
Copyright © 2011 Nelson Education Limited
2-5