TEMPERATURE SCALES
advertisement

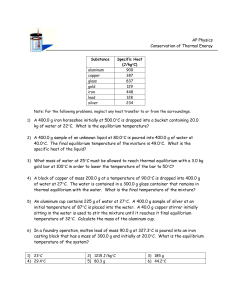
Professor Mason Physics 4B Readout electronics for nuclear applications Silicon and CdZTn pixel detectors Looking at an alarm clock using 20, 35, 60 and 90 keV TEMPERATURE SCALES Given the table above, note the boiling point of water in Fahrenheit and Celsius. Also note the freezing point. Assume that there is a linear relationship between Fahrenheit and Celsius. On your whiteboard draw a graph showing Fahrenheit as a function of Celsius for these two data points. Now assume that our equation is a simple linear relation of the form: Y = mX + b or TC = mTF + b 1. The slope m tells us how many Celsius degrees there are for each Fahrenheit degree. You can find out what m is by comparing T(steam) – T(ice) (or other fixed point temperatures) in both scales. m= ____________________ °C/°F 2. The constant b can be solved for by noting that when the Celsius scale is 0°C (the ice point) the Fahrenheit scale is 32°F. b= ____________________ °C 3. The final relation is: Express the temperature of this room on the Kelvin scale One Calorie of Heat Energy Thermodynamic Definition: 1 cal = Energy needed to raise the temperature of 1 gram of water 1 degree Celsius Heat Equivalent of Energy: 1 cal = 4.186 Joules 1 Watt = J/s Heat Flow Whenever there is a temperature difference between two objects in contact, heat energy will flow from the warmer object to the cooler object until they reach the same temperature. DOES TEMPERATURE TELL THE WHOLE STORY? We know that when a hotter substance comes into thermal contact with a cooler one the temperatures of the two substances change. These temperature changes are easy to observe when the substances can either mix or come into thermal contact with each other. Do the initial temperatures alone allow us to predict the final temperature of the system after the two substances have interacted with each other? Suppose you have two liquids of masses m1 and m2 in thermal contact inside a fairly well-insulated container. Note: For water, 1 ml = 1 cc = 1 g. Activity: Predicting Temperature Changes a. If you were to place two equal masses of the same type of liquid having different temperatures in thermal contact, how would you determine the final temperature? For example, suppose m1 = m2 = 32 g while T1 = 5°C and T2 = 35°C. What would you expect to happen to the temperature of each of the liquids after a while due to thermal contact between them? Note: Assume that the liquids are inside an insulated container so no interaction takes place with the room. Should you add cream to coffee right away? b. If you were to place two different masses of the same type of liquid having different temperatures in thermal contact, how would you determine the final temperature? For example suppose m1 = 50 g and m2 = 200 g while T1 = 5°C and T2 = 30°C. What would you expect to happen to the temperature of each of the liquids after a while due to thermal contact between them? Note: If you can’t make a quantitative prediction, try making a qualitative prediction. For example, will the liquids in both vessels undergo the same temperature change? . Two vessels containing different masses of water at different temperatures. c. Explain the reasons for your answers in parts a. and b. On the basis of what you already know, what do you think is taking place when the two liquids come into thermal contact? Can you describe a possible mechanism for any interactions you might predict? Do you think it’s possible on the basis of knowing just the temperatures, but not the masses, of the two liquids in the containers to predict the final temperatures of the two liquids after they have been in contact? d. Given an Aluminum Can, a Styrofoam cup and a pair of temperature sensors test your prediction in part b. This can be done by filling the aluminum can with a mass, m1, of hot water. This can then be placed in an insulated container with very cold water. Electronic temperature sensors should be placed in each container of water. Monitoring the temperatures on a real time graph for about 3 minutes should be sufficient. Briefly describe what you did and your results. If possible, you should share your results with others. Note: To compensate for the lack of perfect insulation of the system from its surroundings, use about 4 or 5 times as much chilled water in the Styrofoam cup as you have hot water in the aluminum can. Fig. 1.6. Two vessels of water in thermal contact surrounded by an insulator such as a covered Styrofoam cup. e. How did your observations agree with your prediction? Is there any evidence of the liquids mixing together or exchanging matter during the thermal interaction? Is there any visible exchange of matter? Explain any new ideas about how the thermal interaction might be causing the temperatures to change. Parts of any insulated system can be in thermal contact with each other without mixing. If these parts have different temperatures, they will interact until the entire system is at the same temperature. This is a mysterious process, because the interaction that causes temperature changes in two parts of a system can occur without an exchange of matter. You should have noticed from your experiments and those of your classmates that the relative masses of the parts of your thermally isolated system affect the value of the final equilibrium temperature. Thus, the interaction between two parts of a system cannot be explained as a simple temperature exchange. We need to create a new concept to help us understand heating and cooling processes. Scientists have invented the concept of heat to explain this phenomenon. List at least four or five variables that the rate of cooling of an object in a large room might depend on. Describe a situation in which you expect the initial cooling rate of a given object to be rapid and one in which the initial cooling rate might be slow. Note: The initial cooling rate is the change in temperature per second at first and not the total time it takes something to cool. Suppose you placed equal masses of hot tap water into the plastic bottle and aluminum tube. If each container was immersed in a mixture of ice and water, which container would allow the water to cool faster? : In the situation you just studied, the time rate of decrease in temperature of an object is usually proportional to the temperature difference between the object and its surroundings at each time as it cools. This is known as Newton’s Law of Cooling. It can be shown mathematically that the dependence of the temperature, T, of an object as a function of elapsed time, t, is given by: T(t) = (Ti - Ts)e-t + Ts where Ts is the temperature of the surroundings, Ti is the initial temperature of the object and is the cooling constant which depends on a number of factors for a given system. It has dimensions of inverse time. THERMAL CONDUCTIVITY CONDUCTION of HEAT ENERGY: = Heat Flow (SI: J/s = W) k = Thermal Conductivity of the Substance (SI: W/m oC) (The bigger the value of k, the larger the heat flow) A = Cross-sectional Area of Surface Perpendicular to the heat flow (SI: m 2) L = Distance in the medium through which the heat flows (SI: m) Th = Temperature of the hotter end/surface of the substance (SI: oC) Tc = Temperature of the cooler end/surface of the substance (SI: oC) R= L/k = Thermal Resistance or Insulation's R-value. The bigger the R-value, the smaller the heat flow. (SI: (W/m 2 o C)-1) Building Materials' R-value is measured in units of (BTU/hr ft 2 oC)-1. Now make your lab quantitative: Given the can earlier, make a list on your white board of all the things you need to measure in order to determine the Thermal Conductivity of Aluminum. Either using the graph you made previously, or with a new graph, create a graph of Heat vs time using the definition that 1 cal = 4.186 Joules = 1 gram water 1 degree C. This time make sure you have a temperature probe touching the inside of the can, and the other touching the outside of the can. Make a screen shot of this graph for your blog and explain how you determined dQ / dt. Determine the Thermal Conductivity of Aluminum on your whiteboard based on your data. Include an uncertainty estimate with a discussion of how you estimated uncertainty. Take a picture of your group’s whiteboard for your blog. Heat Flow Through Compound Layers RT Hn Rn Tn Th = Heat flow through all layers = Total Therminal Resistance through all layers = Heat Flow through the n th layer = Therminal Resistance of the n th layer = Temperature across the n th layer = Temperature of the surface layer which is hotter Tc = Temperature of the surface layer which is cooler HEAT CONDUCTION PROBLEM Two square bars that have a cross-sectional length of 5.00 cm long are joined end-to-end. One bar is made of copper 26.0 cm long and the other bar is made of aluminum 33.0 cm long. The copper end is placed in boiling water and the aluminum end is placed in an ice-water mixture. If the sides of the bars are well insulated so that no heat is lost out the sides of the bars: (A) What is the heat flow through the copper bar? (B) What is the heat flow through the aluminum bar? (C) What is the temperature at their interface where they are joined? (D) What would have to be the length of the aluminum bar in order for the temperature of the interface to be exactly 50 oC? Sketch and Process: Heat flows through two joined bars due to conduction as a result of a temperature difference across their free ends. Relevant Physics: This is a problem the flow of heat - Heat Conduction. Moreover, this problem involves heat flow through compound layers. The easiest way to solve problems involving layers of material is to find the R-value of each of the layers and add them together to get the effective R-value of the whole system. Since the energy that flows through any one layer must be equal to the energy that flows through any other layer or the system layers as a whole, we can use this effective R-value of the whole system to find the heat flow. Here L is the thickness of each layer and k is its thermal conductive. (A) Find The heat flow through each section must the same or else energy would not be conserved - what flow in must flow out or else there would be a build up heat energy in some section. Therefore, if we find the overall heat flow it must also be to the heat flowing in the copper or the aluminum. (B) Find The heat flow through both bars must be the same and we already found it in part A. If we knew the temperature at the interface of the two metals we could check this result directly by calculating through separately. On to part C. (C) Find the interface temperature TI We could choose to look at the heat flow through either material. Material 1: Solving for the temperature at the interface, TI Material 2: (as a check -- they should be the same) Thus we see that most of the temperature drop is in the copper because copper is not only a better conductor than aluminum (kCu / kAl = 1.66) but it is also shorter. D) Find LAl if TI = 50.0 oC. One again we use the fact that the heat flow through both metals must be the same. Since we know the temperature at the interface we can find the new R-value that the aluminum must have. Not surprisingly, they are the same. Although not stated directly, it is assumed that length of the copper bar does not changed. Thus we can and already have found the R-value for the copper in part A. Since R = L/k we can use this to find the new length of the aluminum that give the same R-value as the copper. Another way to state this is that in order for the interface temperature to be halfway between the two end temperatures, the ratio of the length of copper to the length of the aluminum must be the same as the ratio of their thermal conductivities, kCu / kAl = 1.66. And indeed (26.0 cm)/1.66 = 15.6 cm. HEAT TRANSFER AS AN ENERGY EXCHANGE IS HEAT TRANSFER A PSEUDONYM FOR ENERGY TRANSFER? So far you have made observations that indicate that interactions take place when two substances in thermal contact are at different temperatures. We have dubbed these interactions “heat transfer.” In this Activity Guide and in most textbooks, you are told glibly. What evidence do you have that that heat transfer is a form of thermal energy transfer? You should wrap the nichrome wire loosely around your index finger, attach it to the generator, and have your partner turn the crank. Transferring Heat to Your Finger a. turned? What happens to your wire-wrapped finger when the crank is being . b. Remember the definition of work? Are you exerting a force on the handle as you turn the crank? If so, is the handle moving in the direction of the force you are exerting? Are you doing mechanical work when you turn the crank? Why or why not? c. Is it less work to turn the crank when the wire is not attached to the generator? What happens when a light bulb is attached? d. What is probably happening to the energy you expend doing the work to turn the crank? What types of energy transformations might be taking place when the finger is wrapped with wire? The idea of heat as an energy transfer process that results from temperature differences leads to more formal definitions of heat and of temperature as a measure of thermal equilibrium. These are summarized below. Although we ask you to memorize very few things in this course, these definitions should be memorized and their meanings understood. Retaining these two important thermodynamic concepts is critical to mastering the next unit on the First Law of Thermodynamics, the ideal gas law, and heat engines. 1. Heat is energy in transit between two systems in thermal contact due only to temperature difference with the hotter system losing thermal energy as the cooler system gains it. 2. Two objects are in thermal equilibrium, and hence have the same temperature, if no net energy is exchanged between them when they are placed in thermal contact. Warning: Never turn on a Immersion heater unless the end of the immersion coil is completely submerged in water. Otherwise it will burn out! These Immersion heaters are rated to 300 Watts. However, this may or may not be accurate. Use the Wattmeter to measure the actual output of your immersion heater while it is in water and record it on your board. Power = ________ If you run the heater for 20 seconds, how much heat should you generate? You will place your immersion heater in 200 ml of room temperature water. Measure the initial temperature of the water and record it on your whiteboard. Now start logging temperature data and measure the increase in temperature when you turn on the immersion coil for 20 seconds. Be sure to stir vigorously while the immersion heater is running. Modify your graph so that instead of being temperature vs Heat. HINT: Use the calculation based on the wattage of the heater. Invert the axis so that you are plotting heat vs temperature. Include this graph in your blog Is the relationship, linear, quadratic or something else? If you halve the mass of the water in your cup to 100ml, what effect does this have on your measurement? Write an equation that describes the relationship that you observe. Identify the physical meaning of each of the parameters. Change your graph to plot Heat per Unit Mass vs Temperature. Write in words the physical meaning of the slope of this graph. Include this graph in your blog Repeat this experiment using 100ml of vegetable oil. Find the new slope of this graph. Include this graph in your blog The answers to these questions involve a property of a substance called the specific heat. The constant c that you determined for water and oil is known as the specific heat of a substance. Specific heat is defined in J/kg • °C units as the amount of heat energy in joules needed to raise a kilogram of a substance by one degree Celsius. The mathematical definition of specific heat is c Q m T A material with a high specific heat can have a large quantity of heat energy transferred to it without changing its temperature very much. A material of the same mass with a small heat capacity will undergo the same temperature change when a smaller amount of heat is transferred to it. Heat Flow Whenever there is a temperature difference between two objects in contact, heat energy will flow from the warmer object to the cooler object until they reach the same temperature. If there is no phase change in the substance, the heat energy that flows out of or into a substance can be expressed as: Q = Amount of Heat flowing into or out of one of the substances in contact (SI: J, Other: cal) > 0 Heat flows into the substance < 0 Heat flows out of the substance m = mass of the substance (SI: kg, other: g) c = Specific Heat Capacity of the substance (SI: J/kg/ oC, other: cal/g/ oC) T = T f - T o = Change in Temperature of a substance (SI: K or oC) HEAT CAPACITY (The Storage of Thermal Energy) HEAT CAPACITY of a SYSTEM : (Most General Definition and the one we hardly ever use) CT = Total Amount of Heat Energy required to raise the temperature of some System 1oC. SI: J/ oC For such a a system: Q = CT ΔT We hardly ever use this definition because every system would have a different CT even if it were made of the same substance. For example, a 100g of pure water and 50g of pure water would each have a different total heat capacity. The heat capacity CT is useful for composite systems composed of different substances like a mixture of a fixed amount of water and copper. SPECIFIC HEAT CAPACITY of a SUBSTANCE : (Conventionally used in Physics) C = Amount of Heat Energy per kilogram that is required to raise the temperature of one kilogram of the substance 1oC. SI: J/(kg⋅oC) or J/kg/oC For such a system: Q = m C ΔT MOLAR HEAT CAPACITY of a SUBSTANCE : (Routinely used in Chemistry) c = Amount of Heat Energy per mole that is required to raise the temperature of 6.022x1023 molecules of the substance 1oC. SI: J/(mole⋅oC) or J/mole/oC For such a system: Q = n c ΔT VOLUMETRIC HEAT CAPACITY of a SUBSTANCE : (Commonly used in storage of Solar Energy) cv = Amount of Heat Energy per unit volume that is required to raise the temperature of one cubic meter of the substance 1oC. SI: J/(m3⋅oC) For such a system: Q = V cv ΔT Historically, heat capacities have been expressed in oC rather than K (Kelvin) because most temperature devices measure temperatures in degrees Celsius. Technically, the units of heat capacity should be expressed in degrees K rather than degrees oC. However, ΔT has the same value regardless if T is measured in K or oC since the units of K and oC have the same size. Thus both system of units will give the same answer. ADDING HEAT PROBLEM If 215 kJ of heat energy is added to each of the following substances(s) what will be their final temperature: (A) 1.50 kg of water at 60.0 oC. (B) 14.5 kg of copper at -22.0 oC. (C) 14.5 kg of copper and 1.50 kg of water in thermal contact with each other, both initially at 32.0 oC. What fraction of the added heat energy goes into the water ? (D) 790 g block of ice at -5.00 oC. Sketch and Process: A substance(s) is heated up by certain amount of heat energy. Relevant Physics: When there is no phase change, the heat added can be found using the known values of the specific heat capacities of the substances. If the substance also changes phase, then we must also account for the latent heat during the phase change. The most difficult part of solving these types of problems is trying to decide if the substance changes phase. Some times it is straightforward, other times it is far trickier. If you get an answer that seems unreasonable then its a sure clue that your initial assumption about the phase change might have been incorrect. For example, if you use Q = mcT alone, then your answer should lie some where between the melting point temperature and the boiling point temperature of the substance. (A) Find Tf of the water alone. First let us decide if 215 kJ of heat is enough energy to raise the temperature above the boiling point of water. The energy required to raise the 1.50 kg of water from 60.0 oC up to the boiling point of water (100 oC) is: Since this amount of energy is larger that the given input of 215 kJ, the water will not reach 100 oC. Thus there will be no phase change, but the final temperature should be near the boiling point because 215 kJ is close to the 251 kJ needed to reach 100 oC. To find the final temperature we set Q to 215 kJ and solve for . If you did not check to see if the water would change phase and just used Q = mcT and got 94.2 oC it would be reasonable to assume that the water did not change phase and that your answer was correct. Had your answer been something screwy like a temperature lower that the initial temperature or a temperature above 100oC, then you would know something is amiss. For example, had the input heat energy been above 251,110 J, then your answer would have been above 100 oC if you only used Q = mcT to solve the problem. B) Find Tf for the copper alone. Since the melting point of copper is over 1000 oC (see the Table of Latent Heats), 215 kJ of energy are not going to cause a phase change in 14.5 kg of copper. Using copper's specific heat capacity, Since the specific heat of copper is less than that of water (387 J/kg/oC compared to 4186 J/kg/oC), it is not surprising that the change in temperature of the copper is less than that of the water. It takes 4186/387 = 10.8 times as much heat to raise a kg of water to same temperature as a kg of copper (if they both start at the same temperature). Equivalently, it would take 10.8 times as much copper to have same total heat capacity as water. In this problem there is only 14.5 kg/1.5 kg = 9.67 times as much copper. (C) Find Tf when the water and copper are in thermal contact, and the fraction of heat energy that is absorbed by the water. If 215 kJ of energy cannot cause a phase change in the water by itself or the copper by itself, then there will be no phase change when the heat energy is added to both. The 215 kJ of energy is not shared equally by the two substances because they have different heat capacities. Only if mwcw = mCucCu would they each absorb the same amount of energy. Note that both substances will have the same final temperature since they are in contact with each other. For both substances, This is basically a statement of the conservation of energy. Each substance absorbs a certain amount of heat energy mcT, and the sum must equal the amount that flows into both. The process by which the heat is added is unimportant since the two substances will come to thermal equilibrium with each other. It will not matter if the copper block is heated up first with the 215 kJ and then dropped into the water or if the water and copper are heated up together. Note that the problem assumes that none of the 2.15 kJ of energy is lost during the heating process. Better yet, that the total energy absorbed by both substances is 2.15 kJ even though some additional amount of energy may have been lost during the heating process. Since both substances have the same initial and final temperatures, both substances will have the same change in temperature T. Solving for T. We could check our answer by calculating the heat energy increase in each substance using the answer we just found and seeing if total gain in heat energy sums up to 2.15 kJ. It checks. This last calculation is also useful in finding the fraction of the input energy that goes in to the water. It is just a coincidence that this is approximately half the input energy. Excel lab: PROBLEM 1: POPULATION MODELING Consider a population of 1000 25-year-olds, 500 50-year-olds, and 250 75-year-olds. Suppose that the 25-year olds consist of 500 couples, each of which gives birth to four kids, and that none of the 75-year-olds are alive 25 years later. How many people are alive one generation (for our purposes 25 years) later? How many people are alive twelve generations (for our purposes 300 years) later? What we want to do is use Excel to calculate for us the following chart: Generation Number of 25Number of 50Number of 75year-olds year-olds year-olds 0 1000 500 250 1 2 3 4 5 Total population 1750 (1000+500+250) PROBLEM 2: MODELING FREE FALL WITH AN INITIAL SPEED DOWNWARD Open up a new workbook (File menuNew Workbook) Enter the following in your workbook: A 1 2 3 t 0 B v 5.2 C d D E