AP Physics Conservation of Thermal Energy Substance Specific
AP Physics
Conservation of Thermal Energy
Substance Specific Heat
(J/kg C) aluminum 900 copper glass gold iron
387
837
129
448
1)
2) lead silver
128
234
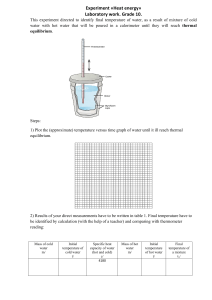
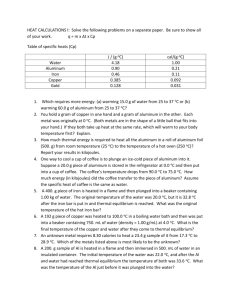
Note: For the following problems, neglect any heat transfer to or from the surroundings.
A 400.0 g iron horseshoe initially at 500.0
C is dropped into a bucket containing 20.0 kg of water at 22 C. What is the equilibrium temperature?
A 400.0 g sample of an unknown liquid at 80.0
C is poured into 400.0 g of water at
40.0
C. The final equilibrium temperature of the mixture is 49.0
C. What is the specific heat of the liquid?
3) What mass of water at 25 C must be allowed to reach thermal equilibrium with a 3.0 kg gold bar at 100 C in order to lower the temperature of the bar to 50 C?
4) A block of copper of mass 200.0 g at a temperature of 90.0
C is dropped into 400.0 g of water at 27 C. The water is contained in a 300.0 g glass container that remains in thermal equilibrium with the water. What is the final temperature of the mixture?
5) An aluminum cup contains 225 g of water at 27 C. A 400.0 g sample of silver at an initial temperature of 87 C is placed into the water. A 40.0 g copper stirrer initially sitting in the water is used to stir the mixture until it reaches it final equilibrium temperature of 32 C. Calculate the mass of the aluminum cup.
6) In a foundry operation, molten lead of mass 90.0 g at 327.3
C is poured into an iron casting block that has a mass of 300.0 g and initially at 20.0
C. What is the equilibrium temperature of the system?
1) 23 C
4) 29.4
C
2) 1215 J/kg C
5) 80.3 g
3) 185 g
6) 44.2
C