Heat Calculations Worksheet: Specific Heat & Energy Transfer
advertisement

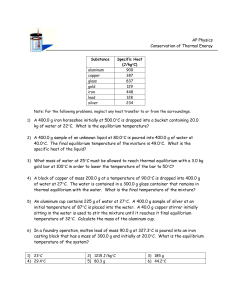
HEAT CALCULATIONS I: Solve the following problems on a separate paper. Be sure to show all of your work. q = m x ∆t x Cp Table of specific heats (Cp) Water Aluminum Iron Copper Gold J / (g∙oC) 4.18 0.90 0.46 0.385 0.128 cal/(g∙oC) 1.00 0.21 0.11 0.092 0.031 1. Which requires more energy: (a) warming 15.0 g of water from 25 to 37 oC or (b) warming 60.0 g of aluminum from 25 to 37 oC? 2. You hold a gram of copper in one hand and a gram of aluminum in the other. Each metal was originally at 0 oC. (Both metals are in the shape of a little ball that fits into your hand.) If they both take up heat at the same rate, which will warm to your body temperature first? Explain. 3. How much thermal energy is required to heat all the aluminum in a roll of aluminum foil (500. g) from room temperature (25 oC) to the temperature of a hot oven (250 oC)? Report your results in kilojoules. 4. One way to cool a cup of coffee is to plunge an ice-cold piece of aluminum into it. Suppose a 20.0 g piece of aluminum is stored in the refrigerator at 0.0 oC and then put into a cup of coffee. The coffee’s temperature drops from 90.0 oC to 75.0 oC. How much energy (in kilojoules) did the coffee transfer to the piece of aluminum? Assume the specific heat of coffee is the same as water. 5. A 400. g piece of iron is heated in a flame and then plunged into a beaker containing 1.00 kg of water. The original temperature of the water was 20.0 oC, but it is 32.8 oC after the iron bar is put in and thermal equilibrium is reached. What was the original temperature of the hot iron bar? 6. A 192 g piece of copper was heated to 100.0 oC in a boiling water bath and then was put into a beaker containing 750. mL of water (density = 1.00 g/mL) at 4.0 oC. What is the final temperature of the copper and water after they come to thermal equilibrium? 7. An unknown metal requires 8.30 calories to heat a 23.4 g sample of it from 17.3 oC to 28.9 oC. Which of the metals listed above is most likely to be the unknown? 8. A 200. g sample of Al is heated in a flame and then immersed in 500. mL of water in an insulated container. The initial temperature of the water was 22.0 oC, and after the Al and water had reached thermal equilibrium the temperature of both was 33.6 oC. What was the temperature of the Al just before it was plunged into the water?