advanced instrumental analysis
advertisement

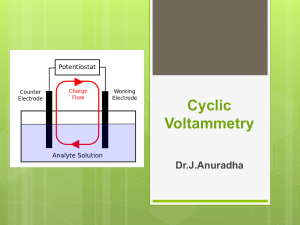
ADVANCED INSTRUMENTAL ANALYSIS 1008-621-85 (031) Fall 2003 Thursdays: 8 AM to 2 PM Instructor: L. Paul Rosenberg Office: A256 Phone:475-6159 e-mail: lprsch@rit.edu OUTLINE: This instrumental analysis laboratory is aimed at giving orientation towards the use of sophisticated modern instruments that are relevant to the growth of the modern science and technology. It is intended to give an opportunity to developing your innovative skills. The approach adopted here is to give you relevant background materials on selected topics. The topics are enclosed herewith for guidance. This list contains instrumental analysis by AA, GC/MS, HPLC, UV-VIS, IR, cyclic voltammetry and Ionselective electrode. The analysis is to be carried out following the procedures given in “Chemistry Experiments for Instrumental Methods.” by D.T. Sawyer et al. The procedure can be modified in appropriate cases to suit the analysis requirements. SCHEDULE-DATES Week 1 (9/11) Week 2 (9/18) Week 3 (9/23) Week 4 (10/2) Week 5 (10/9) Week 6 (10/16) Week 7 (10/23) Week 8 (10/30) Week 9 (11/6) Week 10 (11/13) Week (Finals) EXPERIMENT NO. Orientation-overview EXPERIMENT-1 EXPERIMENT-1 EXPERIMENT-2 EXPERIMENT-2 EXPERIMENT-3 EXPERIMENT-3 EXPERIMENT-4 EXPERIMENT-4 EXPERIMENT-5 No Lab. REPORT DUE DATES Report 1 Report 2 Report 3 Report 4 Report 5 The laboratory report is due one week following its completion. The following experiments are suggested from the above book. 1 Experiment 4-1: Cyclic Voltammetry Experiment 4-2: Study of Electrode Mechanism by Cyclic Voltammetry Experiment 9-3: Det. Of Ca,Fe,Cu in Food by AAS Experiment 12-3: Qualitative Identification of hydrocarbons by GC/MS Experiment 13-5: Separation of carbohydrates by HPLC Experiment 6-1: Spectroscopy in Visible Region::Two component mixtures Experiment 8-2: IR spectra of Aldehydes and Ketones. Experiment : Ion selective electrode-Fluoride ion determination GRADING SCHEME: Lab. report 1 : 20% Lab. report 2 : 20% Lab. report 3 : 20% Lab. report 4 : 20% Lab. report 5 : 20% LABORATORY REPORT WRITING STYLE: The report should be written in the style of a journal article. It should have a) Title b) Abstract c) Introduction d) Experimental e) Results f) Discussion of results g) Conclusions h) Acknowledgment i) References If you wish to have a look at the format, consult any article in the Analytical Chemistry journal; it is easily available in the library. It may also be useful to have a look at the Journal of American Chemical Society as a general reference. to the style. Alternatively, you can follow the style given in the book on “Instrumental Analysis” by D.T. Sawyer et al. The following description will give what is generally expected in the above areas. a) Title: It should be appropriate to the experiment and the results. It should carry key words by which the article could be identified. b) Abstract: Brief summary of the results focusing on the outcome of the analysis. c) Introduction: It should give an outline of the analytical problem being solved with references to the earlier literature. A short description of the technique used 2 and the theoretical principles involved should be given. Don’t discuss the theory extensively. d) Experimental: Discuss the experimental procedures including the instrumentation used. It generally can be divided into chemicals used in the preparation of the reagents (include the purity of the grades of chemicals). Any standardization procedure used should be discussed here. If the solution preparation involved precipitation or coagulation etc., it should be detailed out in the this section.. Instrumentation used in the analysis should be given with procedural details. Overall by reading the experimental section an analytical chemist must be able to reproduce the reported results to a great degree of accuracy. e) Results and discussion: The data collected should be reported in a tabular form. The degree of precision of measurements should be reflected in the data. The units of measurements should be SI units. Figures are appropriate way of showing the nature of the data sampled and the associated trend. Orient discussions of the results with reference to the tables and figures. The data obtained should be discussed with respect to the literature reports by giving appropriate references. f) Conclusions: It should be a short paragraph giving information on the final conclusions drawn from the experiment. If the conclusions warrant future work, it should also be discussed. g) Acknowledgment: In carrying out the experiment if you have sought the help of others, you should acknowledge here. h) References: Follow the journal pattern- look into Analytical chemistry or Journal of the American Chemical Society. GRADING SCHEME: (Total 100 points) 1. Title page 2. Abstract 3. Introduction & Theory 4. Experimental 5. Results and discussion 6. Conclusions 7. References 5 points 10 points 15 points 10 points 35 points (10 points for Questions) 15 ponts 10 points Guidelines: 3 Prepare to carry out the standard analysis on the instrument before starting on the problem. Wear safety glasses all the time in the lab. Do not do the experiments alone. Do the experiments within the scheduled period. Manuals for the use of the instruments are available and read them before starting the experiment. Instrumental failures or trouble should be reported immediately. Feel free to ask questions. Available instruments: UV/Vis spectrometer High performance liquid chromatography (HPLC) Gas Chromatography (GC) Gas Chromatography/mass spectrometer (GC/MS) Atomic absorption spectrometer (AA) Fourier Transform Infrared Spectrometer (FT-IR) Nuclear Magnetic Resonance Spectrometer (NMR) Cyclic Voltammetry (CV) Ion-Selective Electrode: Fluoride and Lead Ion Selective Electrodes 4