Biochemistry Review
advertisement

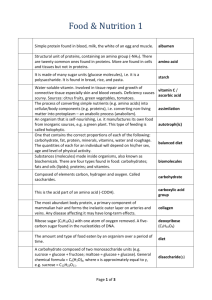
Biochemistry Review 1. What is an organic compound? A compound containing Carbon, comes from living organisms, usually large and complex 2. How many/what types of bonds does carbon form? 3. What is a functional group? 4. What happens during condensation? This is the process of joining 2 monomers together by removing a water molecule for every bond that is formed 5. What happens during hydrolysis? This is the process of taking apart a polymer by using one water molecule to break every bond. 6. What is the difference between structural formulas and molecular formulas? Structural formulas show the compound’s arrangement while a molecular formula is just the number and types of elements used to make the compound 7. What is an isomer? An isomer are when 2 or more compounds have the same molecular formulas but have different structural formulas. EX: glucose and fructose are both C6H12O6 but when they are drawn they have different shapes. 8. What is a carbohydrate? A molecule containing Carbon, Hydrogen and Oxygen – sugars and starches 9. What are some foods that are high in carbs? Bread, pasta, potatoes, candy, desserts, bread, sugar, honey 10. What letters do carbs usually end with? ose 11. What are carbs used for? Quick energy, the body’s #1 fuel 12. What is a monosaccharide? a simple sugar 13. What is the formula for a monosaccharide? C6H12O6 14. What type of energy do we get from monosaccharides? Quick energy 15. What are the 3 types of monosaccharides? Glucose, fructose, galactose 16. What monosaccharide is the most important? Glucose Why? 1st choice of carb and it’s the only type that the brain cells can use. 17. Where is fructose found? In fruit Why is it different? Tastes sweeter than the other monosaccharides 18. Study your picture page….glucose, galactose and fructose 19. What is a disaccharide? A double sugar, formed by joining 2 monosaccharides together 20. What is the formula for a disaccharide? C12H22O11 Why is this formula not double a monosaccharide? you must remove a water due to the condensation reaction that bonds the sugars together 21. Name the 3 disaccharides? Where is each found? What are the components of each? a. Sucrose – table sugar – glucose + fructose b. Lactose – milk sugar – glucose + galactose c. Maltose – brewer’s sugar – glucose + glucose 22. Study your picture page 23. What is the name of the bond? Glycosidic linkage 24. What is a polysaccharide? A complex carbohydrate – made by joining lots of monosaccharides together 25. What are the 3 polysaccharides? Where would each be found? a. Starch – found in plants – how they store excess sugar – foods such as grains, breads, pastas are high in starch b. Glycogen – found in animals – how they store excess sugar – stored in muscles and liver tissue c. Cellulose – found in plant cell walls – allows them to be rigid, grow tall 26. Which type of polysaccharide cannot be digested by humans for energy? Cellulose cannot be digested for energy because humans are missing an enzyme Why do we eat this type? We eat foods containing cellulose because they are a good source of fiber, vitamins and minerals. 27. Picture page 28. Simple carbs and complex carbs? Simple carbs are sugars (mono and disaccharides) they give a quick spike of energy because they are easy to break down. Complex carbs are the polysaccharides – starches and fiber. They take longer to break apart and use so they provide energy for a longer period of time. Monosaccharides and disaccharides are simple carbs – they are burned very quickly and produce a large spike in energy level which is then followed by a large crash. Polysaccharides are complex carbs and take longer to burn – they burn at a steady rate and create a steady, constant level of energy. By loading up on complex carbs (carb loading) before events athletes can stockpile glycogen which they will use during the event…when their glycogen is depleted they will then need a mono/disaccharide for a quick burst of energy – ex – eating a piece of candy or fruit during the marathon. 29. What is a lipid? An organic compound with lots of Carbon and Hydrogen and very few Oxygen 30. How do lipids react in water? They are insoluble, do not mix 31. What are the uses of lipids? Main energy storage molecule – stores it for the long term, waterproofing, buoyancy, cell membranes, insulation, padding around organs. 32. What the monomers of lipids? Fatty acids and glycerol 33. What is a saturated fat? Has all single bonds, solid at room temp, bad for health examples? Butter, lard, meat fat What are some 34. What is an monounsaturated fat? Has some one double bonds, liquid at room temps, good for your health, What are some examples? Olive oil 35. What is a polyunsaturated fat? Has some several double bonds, liquid at room temps, okay for your health, What are some examples? peanut oil, corn oil, canola oil 36. What are omega-3 fats? Healthiest type of fat, helps with many functions in the body found? Found in fish (salmon, tuna) and walnuts Where are they 37. What are trans fats? Are chemically processed fats, the worst type of fats for your health. Where are they found? Are found in baked goods, snack foods, fried foods – many foods are not removing them. 38. Rank the fats #1 omega, #2 monounsaturated, #3 polyunsaturated, #4 saturated, #5 trans-fats 39. Healthy fats – the omega-3 and monounsaturated, found in fish, nuts and olives, needed for healthy skin, hair, cell membranes 40. What percent 30% 41. What is a triglyceride? A lipid that has 3 fatty acids and 1 glycerol 42. Name of bond? Ester bond 43. What are waxes used for? Used for waterproofing – plants and animals 44. Where are steroids found naturally? Hormones, nerve tissue, poisons and venom 45. Where is cholesterol? Type of lipid that can build up in the blood vessels 46. The two types? HDL and LDL. What is different? HDL is a good cholesterol and LDL is the bad cholesterol that clogs arteries and sticks to the walls of blood vessels. What does it causes when you have too much? Causes blocked arteirs wich can lead to heart attacks or strokes 47. What is caused by excessive bad cholesterol? strokes and heart attacks 48. What are proteins? Organic compounds made of Carbon, hydrogen, oxygen, nitrogen and sulfur 49. What are the 2 main functions of a protein? Structural building materials for the body and functional chemical compounds (enzymes) 50. Where are some places that proteins can be found? Hair, skin, nails, cartilage, muscles, fur, cartilage, bones 51. What are some foods high in proteins? Meats, eggs, cheese, nuts 52. What is the monomer of protein? Amino acids (aa) 53. How many amino acids are there? 20 different ones 54. What makes each different? The radical group – changes with each type of amino acid – there are 20 different radicals 55. What is the difference between an essential and nonessential amino acid? Essential aa must be obtained through food because the body cannot make them. Nonessential can be made by the body. 56. How does our body use the protein we eat? Breaks the proteins apart into the individual amino acids and then uses those amino acids to build new proteins according to the directions of the DNA 57. What is the bond between amino acids called? Peptide bond 58. Recognize picture 59. Each individual is unique because of their _PROTEINS____ which are determined by their __DNA _____. 60. What is an enzyme? a protein that acts as a catalyst 61. What does an enzyme do for a reaction? It speeds up the rate of the reaction by lowering the amount of activation energy needed to start. 62. What does it mean that enzymes are specific? They will only work on one type of reaction or on one compound. They can be compared to a key – specific to the lock, used many times without damaging Enzyme – specific to the reaction, can be reused many times by the body for that reaction 63. What is a nucleic acid? A master molecule of the cell 64. What is the monomer of a nucleic acid? Nucleotide 65. What are the 2 types of nucleic acids? DNA (stores the information) and RNA (transfers the information) 66. What are the 3 parts of a nucleotide? Phosphate, sugar and nitrogen base 67. picture page