BIOLOGICAL MOLECULES
advertisement

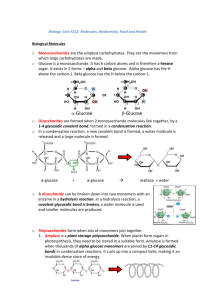
BIOLOGICAL MOLECULES Molecular biology is the study of the structure and function of biological molecules. Metabolism is all of the biochemical reactions in the body. Many biological molecules are made up of many similar or identical monomer subunits and these are called polymers. Polysaccharides and proteins are polymers. The bonds within polymers can be broken by splitting with water. These are hydrolysis reactions. Bonds are formed by removing water during condensation reactions. Carbohydrates have the general formula C (H O) . They can be divided into the monosaccharides, disaccharides and polysaccharides. Monosaccharides are sugars, sweet and dissolve in water. They have the general formula (CH O) . Trioses have n = 3, pentoses n = 5 and in hexoses n = 6. Glucose has the molecular formula C H O . The arrangement of atoms can be drawn in a structural formula. Glucose can have ring structures. There are 2 isomers of glucose which are alpha and beta. Beta glucose has the H pointing down on carbon one – remember HB pencils. A monosaccharide such as glucose or fructose is important as an energy source as it has lots of carbon – hydrogen bonds. When they are broken they release a lot of energy. They are also important as building blocks to make larger molecules. KNOW THE STRUCTURE OF THE GLUCOSE ISOMERS. alpha glucose If 2 alpha glucose molecules join in a condensation reaction, a disaccharide forms with a glycosidic bond between them. LEARN THIS DIAGRAM. 3 examples of disaccharides are sucrose, maltose and lactose. If many monosaccharides join a polysaccharide forms. Starch is an example, where many alpha glucose molecules are joined. It is a mixture of amylose and amylopectin. Amylose is a long unbranching chain with many 1,4 links. Amylopectin is a smaller branching chain with both 1,4 and 1,6 links. Both are found together coiled into compact starch grains. This allows a lot of them to be stored. Starch can be easily broken down to release glucose for energy. It is insoluble allowing for storage and not changing the water potential of plant cell contents and upsetting osmosis. is the energy storage carbohydrate of animal liver and muscle cells. It is like amylopectin but even more branched. Cellulose is a polymer of beta glucose. In order for the glucose molecules to join each successive molecule must be rotated 180 degrees. The hydrogen atoms of the OH groups are weakly attracted to oxygen atoms in the same cellulose molecule and in neighbouring molecules. Many of these hydrogen bonds together give great tensile strength. 60 – 70 molecules form microfibrils. These are held in bundles by hydrogen bonds into fibres. To increase strength a cell wall has layers of fibres running in different directions and a glue like matrix. Cellulose is insoluble – the cell wall would break up otherwise in water. Cellulose fibres are permeable between the molecules so allow passage of substances to and from the plasma membrane. RECOGNISE DIAGRAMS OF AMYLOSE, AMYLOPECTIN AND CELLULOSE. Lipids are a diverse group. The most common are the triglycerides (fats and oils). They are formed from a glycerol and 3 fatty acid molecules that join after condensation reactions by ester bonds. LEARN THE DIAGRAM OF THIS REACTION. Lipids are insoluble in water, non-polar and hydrophobic. Unsaturated fatty acids contain double bonds and this makes them melt more easily. They are usually oils. Polysaturated lipids have many double bonds, monosaturated have one and saturated (animal fats) have none. They are good stores of energy, providing about 2.5 times more energy per gram compared to carbohydrates. They have many more C-H bonds. Fat under our skin gives insulation and provides protection to some organs e.g. the kidneys. Phospholipids are similar to triglycerides but one fatty acid is replaced by a phosphate group. LEARN THE DIAGRAM OF A PHOSPHOLIPID. The phosphate group is hydrophilic (water loving) and the fatty acids hydrophobic (water hating). If shaken with water phospholipids can form bilayers that are the basis of membranes.( See membrane notes) Proteins are very important. They are found in membranes, skin bone and tendons, and haemoglobin, antibodies and enzymes are proteins. They are polymers of amino acids. All amino acids have a general structure with a central carbon bonded to an amine group –NH , a hydrogen, carboxylic acid group –COOH and a side group R. LEARN THIS DIAGRAM. The R group varies in different amino acids and there are 20 naturally occurring amino acids. A -OH from the carboxylic group and a –H from an amine group join in a condensation reaction to form a peptide bond. Two amino acids form a dipeptide and many joined make a polypeptide. LEARN THIS DIAGRAM. The primary structure of a protein refers to the order of amino acids in a sequence. There are many different possibilities in the order to give different proteins. The secondary structure can form when the primary structure twists into an alpha helix held by H bonds or a beta pleated sheet. H bonds are easily broken by high temperatures and pH changes. The protein can coil into a 3D shape which is its tertiary structure. The structure can be held in shape by H bonds, disulphide bridges, ionic bonds and / or hydrophobic interactions. Disulphide bonds are the last to break when heated. Ionic bonds can be broken by pH changes. A quaternary structure is made up of 2 or more polypeptide chains held together by the same bonds as in the tertiary structure. Proteins are globular or fibrous. Globular proteins are roughly spherical, mostly soluble, of tertiary or quaternary structure and metabolically active. Haemoglobin is an example. It is a quaternary structure made up of 2 alpha chains and 2 beta chains. The hydrophobic R groups point in and the hydrophilic ones point out - thus haemoglobin is soluble. The hydrophobic R groups help maintain the 3D shape. Each polypeptide contains a haem group with an iron ion Fe . One oxygen molecule can bind with an iron ion. A haemoglobin molecule can therefore carry 8 O atoms. The haem group is not made of amino acids and this is called a prosthetic group. Collagen is a fibrous protein found in skin, blood vessels and tendons. It is a structural protein and a collagen molecule is made up of 3 polypeptide chains, each in the shape of a helix twisted around to form 3D ‘rope’. Nearly every third amino acid is glycine that is small. This allows the strands to lie close together, held by H bonds that give it strength. Many of these triple helixes lie side by side and are linked by covalent cross-links between the carboxyl end of one molecule and the amine group of another. They are staggered, which gives them more tensile strength. Many molecules make a fibril and many fibrils make a fibre. RECOGNISE COLLAGEN. DIAGRAMS OF HAEMOGLOBIN AND Inorganic ions are charged particles needed by living things. For example calcium phosphate is needed for teeth and bone structure. Ca are needed for the passage of electrical impulses across synapses and muscle contraction. Na and K are needed for nerve impulse transmission along neurons. Mg are needed for chlorophyll structure and the enzyme ATPase has them at their active sites. Fe is found in haemoglobin for oxygen transport.