Correct response
advertisement

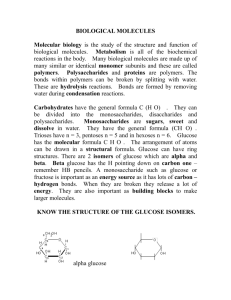
Multiple Choice Review- Large Biological Molecules 1. Why is information about carbon critical to understanding the “molecules of life”? a. it is the backbone of biological molecules required for life b. it is the only element that can form triple bonds c. it results in the theory of vitalism (organic molecules are produced only in living organisms) d. it is able to cause dehydration synthesis 2. Organic chemistry is the study of compounds containing _________ a. carbon and hydrogen b. carbon and helium c. carbon and nitrogen d. hydrogen and nitrogen 3. What is the characteristic of carbon atoms that most contributes to its importance to critical biological molecules? a. the ability to bond with eight (8) other atoms b. the ability to form hydrogen, carbon and covalent bonds c. the ability to choose the type of molecule to produce d. the ability to form 4 (four) bonds producing a 3D structure 4. Why are fossil fuels, such as gasoline, considered organic compounds? a. they contain both hydrogen and carbon b. they contain hydrogen c. they contain carbon d. they are only produced from living organisms 5. There are two forms of hydrocarbons. Which is most able to accept new atoms and why? a. saturated hydrocarbons; they have double or triple bonds that can be broken b. saturated hydrocarbon; they have single bonds only which are easier to break c. unsaturated hydrocarbons; they have double or triple bonds that can be broken d. unsaturated hydrocarbons; they have single bonds only which are easier to break 6. There are many different types of proteins created by bonding amino acids together. How is this possible when there is a small set of amino acids? a. each protein is composed of 1-2 unique monomers b. each protein has exactly the same monomers c. each protein is an arrangement of monomers in a unique manner d. each protein acts differently depending upon the organism www.njctl.org Biology Large Biological Molecules 7. Monomers are bonded together by which of the following processes? a. hydrolysis b. non-hydration lysis c. ionic bonding d. dehydration synthesis 8. Which of the following lists correctly identifies the characteristic structures within an amino acid? a. ammonia - carbon group - side chain b. NH3 - COOH - side chain c. NO2 - COH - side chain d. N2OH- COOH - side chain 9. When proteins are formed the _____ and of one amino acid combines with the _______ end of a second amino acid for form a ___________ a. acid; amine; monopeptide b. amine; amine; polypeptide c. amine; acid; monopeptide d. acid; amine; polypeptide 10. There are 20 standard amino acids. How do they differ from each other? a. the amine groups can vary b. the carboxyl groups can vary c. the carbons can vary d. the side chains can vary 11. Which of the following statements best describes the impact of the structure of proteins? a. shape is driven by chemistry; shape dictates function b. shape is driven by chemistry; polarity dictates function c. shape is driven by environment; environment dictates function d. shape is driven by chemistry; environment dictates function 12. For which level of structure in proteins do the side chains play the biggest role? a. secondary b. tertiary c. primary d. quaternary 13. How does a protein change during denaturation and why is this important? a. the protein loses amino acids; the polarity may be changed b. the protein loses its shape; the protein cannot function c. the protein loses HOH; dehydration synthesis cannot occur d. the protein gains amino acids; the protein changes into a different protein www.njctl.org Biology Large biomolecules 14. Proteins play many critical roles in organisms. Which of the following pairs correctly connects the function of a protein to its class? a. cytoskeleton is structural; antibodies are defense b. speed regulation is enzymes; muscles are hormonal c. hair is storage; hemoglobin is transport d. hormones are signaling; membrane proteins are enzymes 15. Carbohydrates consist of carbon, hydrogen and oxygen. Which of the following represents the general formula for carbohydrates? a. CxH2xOx b. CO2xH c. CxHxOx d. C2xHxO2x 16. The monomer of polysaccharides is a _____________. Table sugar is an example of a __________________. a. monocarbohydrate; dicarbohydrate b. disaccharide; disaccharide c. monosaccharide; disaccharide d. monosaccharide; monosaccharide 17. When comparing proteins and carbohydrates, the following similarities can be identified: a. both consist of linked monomers via the process of hydrolysis b. both consist of linked monomers via the process of dehydration synthesis c. both consist of linked amino acids via the process of hydrolysis d. both consist of linked sugars via the process of dehydration synthesis. 18. Three types of polysaccharides are particularly important to living organisms. They each perform functions vital to cells. Which of the following correctly identifies two of these types of polysaccharides and their primary functions? a. starch, storage; glucose, energy b. starch, cell walls; glucose, storage c. glucose, cell walls; cellulose, energy d. cellulose, storage by animals; glucose, storage by plants 19. One type of carbohydrate can be seen in the image below. How does its structure reflect its function? a. This image is of glucose as its long chain molecules reflect its use for storage b. This image is of starch as its long chains allows for long term storage c. This image is of glycogen as the cross chains reflect its use by muscles. d. This image is of cellulose as its structure reflects its strength www.njctl.org Biology Large Biological Molecules 20. Nucleic acids, such as RNA, consist of monomers of __________________. Each of these monomers consists of __________, ____________, and _______. a. amino acids; side chain, carboxyl group, glucose b. nucleotides; side chain, sugar, nitrate group c. nucleotides; sugar, nitrogenous base, phosphate group d. amino acids; sugar, nitrogenous base, phosphate group 21. There are five types of nitrogenous bases, four of which are found within DNA molecules. These are: a. adenine, thymine, guanine, uracil b. adenine, uracil, guanine, cytosine c. adenine, thymine, cytosine, guanine d. adenine, uracil, phosphate, ribose 22. Pair bonding occurs in DNA between purine molecules and pyrimidine molecules. Why must this type of pairing take place? a. The genetic sequence is found on the pyrimidine bases, so there must be a pyrimidine in each step of the DNA ladder b. The number of hydrogen bonds between the bases must “match” in order for the helix to be double stranded. c. The phosphate bonds required to hold each single strand together must match up in order to produce the double helix. d. The double strand is held together by peptide bonds, which allows for the genetic code. 23. Which of the following best describes the form and function of one type of nucleic acid? a. RNA, a double helix, functions primarily as an archive of genetic information. b. RNA, a single helix, functions primarily as an archive of genetic information. c. DNA, a single helix, functions primarily as an archive of genetic information. d. DNA, a double helix, functions primarily as an archive of genetic information. 24. The nucleotides of DNA form ___________________ bonds. _____________ bonds with guanine and _________________ bonds with ________________. a. oxygen; cytosine, adenine, uracil b. hydrogen; guanine, adenine, cytosine c. hydrogen; cytosine, adenine, thymine d. helium; cytosine, adenine, thymine 25. Molecules may be hydrophobic, hydrophilic or amphiphilic. Lipids are either _______________ or ____________. a. hydrophilic, amphiphilic b. hydrophobic, amphiphilic c. hydrophobic, hydrophilic d. hydrophilic, hydrophilic www.njctl.org Biology Large biomolecules 26. Fatty acids contain ___________-hydrogen bonds, making them ____________. The phosphate “head” in a phospholipid is ______________. The entire phospholipid molecule, therefore, is _______________. a. carbon; hydrophobic; hydrophilic; amphiphilic b. hydrogen; hydrophilic; hydrophilic; amphiphilic c. carbon; hydrophilic; hydrophilic; hydrophobic d. hydrogen; hydrophobic; hydrophobic; hydrophobic 27. The image below represents a type of lipid molecule. It is identifiable by two characteristic smaller molecules. Identify the lipid and the smaller molecules. a. b. c. d. http://www.green-planet-solar-energy.com biglyceride; glucose, fatty acids triglyceride; glycerol, fatty acids triglyceride; glycerol, phospholipid fatty acid; glucose; triglyceride 28. Image #1 below is of a(n) __________________ fatty acid, identifiable because of its _______________ bond(s). Image #2 below is of a(n) ________________ fatty acid because of its ______________ bond(s). Image #1 Image #2 http://thescienceoffat.blogspot.com/ a. b. c. d. saturated fat; double; unsaturated fat; single saturated fat; single; unsaturated; double unsaturated; double; saturated; single unsaturated, single; saturated; double www.njctl.org Biology Large Biological Molecules 29. Soaps and detergents are able to remove oil, grease, etc from items because the _________________ end of the soap molecule bonds with the stains while the “other” end of the soap molecule bonds with ___________. a. hydrophobic; water b. hydrophobic; stains c. hydrophilic; water d. hydrophilic; stains Use the table of results below to answer questions 30, 31 and 32. Results from an experiment testing for presence of specific chemicals. Indicators used are listed across the top of the chart. unknown Lugol’s iodine Sudan stain Biuret reagent 1 positive negative negative 2 negative positive negative 3 negative negative positive 30. The results of the lab tests performed (shown in the data table above) indicate that unknown #1 contains _______________. This is because the _________________ changed from light brown to blue black during the test. a. proteins; Lugol’s b. sugars; Biuret c. starch; Lugol’s d. lipids; Lugol’s 31. The results of the lab tests performed (shown in the data table above) indicate that unknown #2 contains _______________. a. proteins b. glucose c. starch d. lipids 32. The results of the lab tests performed (shown in the data table above) indicate that unknown #3 contains _______________. This is because the _________________ changed from blue to purple during the test. a. proteins; Sudan b. glucose; Biuret c. starch; Biuret d. proteins; Biuret www.njctl.org Biology Large biomolecules 33. We can identify whether or not a lipid is saturated or unsaturated, by its physical state. Unsaturated fats are ______________ and saturated fats are ___________ . a. solid, liquid b. liquid, solid c. liquid, less dense liquid d. unbreakable, solid 34. Waxes and steroids are both considered to be _____________. a. proteins b. carbohydrates c. lipids d. nucleic acids 35. Protein, carbohydrate, and nucleic acid molecules are the result of smaller molecules bonded together. The process that occurs to attach these smaller molecules to one another is ________________ . a. hydrolysis b. dehydration lysis c. hydrosynthesis d. dehydration synthesis 36. Which protein structural level would be least affected by disruptions in the hydrogen-bonding process? a. primary b. secondary c. tertiary d. quaternary 37. RNA and DNA differ in that they utilize different ___________ within their nucleotides. RNA utilizes ____________ and DNA utilizes ____________. a. phosphates; peptide, amino acids b. sugars; deoxyribose, ribose c. sugars; ribose, deoxyribose d. R groups; glucose, galactose www.njctl.org Biology Large Biological Molecules 38. The image below is of a type of biomolecule critical for life. Identify both the type of biomolecule and the specific name of the molecule. #3 http://www.wpclipart.com/science/atoms_molecules/molecules a. b. c. d. protein; antibodies carbohydrate; sucrose carbohydrate; glucose nucleic acid; deoxyribonucleic acid 39. The image below is of a large biomolecule. Identify this biomolecules and its components by selecting the correct list from the choices below: a. RNA; #1 represents the sugar, #2 represents the phosphate, #3 represents the bases b. DNA; #1 represents the sugar, #2 represents the phosphate, #3 represents the bases c. RNA; #1 represents the sugar, #2 represents the bases, #3 represents the phosphate d. DNA; #1 represents the phosphate, #2 represents the sugar, #3 represents the bases #3 #2 #1 http://www.biology-online.org/1/5 Unit 2 - Multiple choice - answer key Question # Correct response Question # correct response 1 a 21 c 2 a 22 b 3 d 23 d 4 a 24 c www.njctl.org Biology Large biomolecules Question # Correct response Question # correct response 5 c 25 b 6 c 26 a 7 d 27 b 8 b 28 c 9 d 29 a 10 d 30 c 11 a 31 d 12 b 32 d 13 b 33 b 14 a 34 c 15 a 35 d 16 c 36 a 17 b 37 c 18 a 38 b 19 d 39 b 20 c 40 www.njctl.org Biology Large Biological Molecules