An assumption that is often implicit or even explicit in much lay (and
advertisement

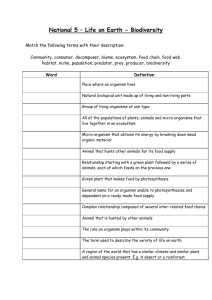
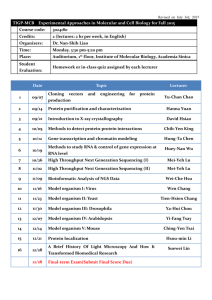
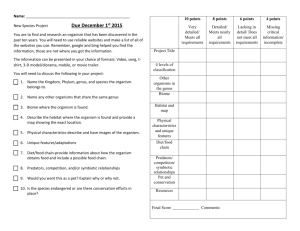
Complexity’s Measure Introduction In biology and the philosophy of biology, the term “complexity” is normally used in one of two ways. It might be used to express (in an information-theoretic manner) how much incompressible information some entity contains. I am not concerned with this usage in this essay, and I have my doubts about how pragmatically useful this approach can be. Alternatively, the term is used when we want to express a less mathematical opinion about the overall or relative complexity of some thing, which is not necessarily biological (although I am concerned only with biological complexity). For example, you or I might say that a lion is less complex than a chimpanzee. In this second situation complexity is almost always defined either very loosely (Dawkins, 1986, ch1) – “I’ll know it when I see it” – or not at all. Sometimes, several definitions are mooted and none settled upon, as Sterelny and Griffiths (1999, p280-282) discuss. At other times a very morphologicallyconstrained definition of biological complexity is used. Furthermore, there is often the assumption that humans are at some peak of complexity. This can be seen in the typical diagram of evolution which shows trilobites somewhere near the bottom of the image and a man striding purposefully across the beach somewhere at the top. This assumption is certainly also implicit in what Edmonds (1999, p2) wrote: “…the starting point of evolution is simple, as it is amenable to a reductionist approach, while the end point (us) is complex.” (my italicisation). Such a claim is a very bold one, particularly given how complexity is left very subjectively defined1. In this essay I hope to develop a systematic approach and definition of biological complexity that is more independent of any anthropomorphic biases we have. By clarifying exactly what it is we are talking about, the approach that will be developed will help to provide structure and precision to any discussion we have which involves complexity. In addition, information about the ways in which an organism is complex can provide historical insights about the kinds of environments the organism’s species evolved in, as discussed by Godfrey-Smith (1996). I would first like to look very briefly at the two most important questions surrounding any decision about a definition of complexity. Within the context of these questions I will evaluate a genotypic model of complexity, which (on the spectrum of possible models) sits at the narrowest extreme. When using such a model the complexity of an organism is nothing more than the length of its genome (Ridley, 2000, p30 and implicit in much of Dawkins, 1976). Once I have described this kind of narrow model I will discuss an intermediate, morphological approach and then close with the development of an alternative and fuller approach to defining and measuring complexity. This approach will occupy the opposite end of the spectrum to the genotypic models, and the genotype will not be considered explicitly. Instead the focus will be on factors such as “structural and behavioural complexity” (Ridley, 2000, p29) and the way the organisms engineer their environment. Edmond defines it as the property of something which makes it difficult to predict – this is obviously of little use, especially when the term “difficult” is not given any more useful meaning! 1 -1- Two Important Questions Examining a claim about the complexity of an organism (either a specific organism or more usefully, the often implicit average member of a species – for example, “Humans are a complex species”) immediately prompts two important questions: What elements should we look at when drawing the boundaries of the organism whose complexity we want to evaluate? What is complexity? I will address these questions in an as species-neutral fashion as possible, to facilitate comparing the complexity of organisms which are from different species. Comparisons between organisms from difference species are the most common and useful form of comparison. This is because it’s difficult to make any meaningful claims about complexity that are not relative to something else and when comparing members of the same species the answer will be “equal complexity” in all but the most tortured cases. Note that the questions just listed are very interrelated and the answers to them will be intertwined. Just the Genes At the tightest end of the spectrum only the genotype is considered as it is argued that this expresses all of a species complexity and that measuring it in other ways as well or instead would either count the same thing twice (over-counting) or not count everything (incompleteness) (Ridley, 2000, p30-31). The genotype can be seen as either the number of base pairs which code for proteins or as the number of genes. Although DNA can do more than just code for proteins, such as control replication and transcription, these other functions will not be considered in this essay. That is because these regulatory sequences only serve to control how the DNA is copied, and since all cells in the organism always have at least one full copy of the organism’s DNA such regulatory sequences cannot contribute in any meaningful way to the complexity of an organism2. The term “gene” must be defined in quite a restrictive sense as well (as the stretch of genome which codes for a particular protein) if the boundaries are drawn so tightly though. This is because a more phenotypic definition – e.g. “the gene for green eyes” or “the gene for olfactorily detecting infidelity” – is actually a position at the other, broad end of the spectrum with the words “the gene for” prepended. Given these definitions, the number of active base pairs which are transcribed as proteins and the number of genes are effectively equivalent definitions and will be treated as one and the same. This is because the same length of DNA (3 base pairs) is only and always needed to code for a protein. The key attraction this definition of an organism’s complexity has is that it is easily measurable: you can just count the number of genes or active base pairs. However, this model suffers from at least 3 problems, which I will now outline. If only the genome and not the developmental environment is being considered then the fact that (as Dawkins, 1982, p14 wrote) “[a] gene ‘for’ A in environment ‘X’ may well 2 Unless of course we are looking to examine the complexity of an organism's cellular or organism-level reproductive process. This is quite a narrow domain and is not the focus of this essay. -2- turn out to be a gene for B in environment Y” becomes problematic. This is because the developmental environment can itself be extremely complex (e.g. Sterelny, Griffiths, 1999, p96) and it cannot be measured in the simple way the length of the genome can be. Indeed, higher level, conceptual approaches are needed, which nullifies the simplicity and objectivity a genotypic model view might appear to offer over higher level, conceptual approaches. It appears that there are two ways this objection could be responded to. The developmental environment could be assumed to be a certain one, or the developmental environment could be somehow included in the definition of the gene. Of these two approaches we have already ruled out the latter as removing the advantages a genotypic model conveys (and the need for such an ad hoc band aid probably also introduces further excessive subjectivity). The former – assuming the developmental environment is a certain one - is just too simplistic though, as discussed by Sterelny and Griffiths, 1999: “[a] trait would be literally genetically determined if it could not be altered by changing nongenetic factors, a situation that we can be sure never arises.” It would therefore seem that the developmental environment poses a significant problem for the types of model which are fundamentally genotypic. Secondly, a gene has only a very indirect impact on the morphological or behavioural complexity of an organism. This is because a gene (as it must be defined by someone who wants to use such a model) codes only for proteins. Hence anybody who draws the boundaries so tightly must identify a single protein, the presence of which causes some (probably macroscopic, but not necessarily) complexity-increasing trait3. Alternatively, a monotropic set of genes could take the place of a single gene. A monotropic set of genes is unlikely but theoretically possible. Such a set of genes would code for proteins which affect just one phenotypic trait, regardless of what alleles are present. An example of such a possibility is a gene or set which codes for proteins which only affect the pattern of markings on an animal’s coat. This is perhaps the most plausible possibility, but it is important to remember that in many species every organism has exactly the same alleles over long stretches of the active part of its genome which codes for proteins, and that a vast number of proteins (and therefore genes) are usually involved in the expression of even the simplest traits. In other words, finding a set of genes which wholly and only code for just one trait is very unlikely, and genotypic models assume that such sets occur frequently (even always). If such a gene or set of genes cannot be regularly and easily found then it means that drawing a meaningful link between a definition of complexity related to some organism’s traits (which is what is sought to make possible the comparisons we are concerned with in this essay) and the number of genes or length of the genome is tentative at best. This leads me to conclude that drawing the boundary of an organism at the edge of its genome is drawing it too tightly. In addition to having a very indirect impact, it is also the case that because genes frequently do interact there may not be a positive correlation between the length of the genome and the organism’s complexity (unless you are calling the length of the genome “complexity”, at which point the whole exercise becomes farcical). At best the Such a trait could be morphological – an eye, a type of cell or even an organelle – or behavioural, such as a cockroach’s response to changing air-pressure. 3 -3- relationship between the length and the interactions and consequential complexity may be roughly exponential, but of course the exponent could be dramatically different for different species. Consider wheat (16.5 billion base pairs) and humans (3 billion base pairs). While these are the actual number of base pairs and not the number of active base pairs (about 2% in humans) it is important to note just how less complex wheat seems than us, even when its biology is studied to the same level of detail as human biology is. If only one fifth as much of the wheat genome was active though – 0.4% – that would indicate that wheat was as complex as us, and much more complex than many other seemingly more complex organisms like fruit flies. Edmond (1999, p4) suggests that the genotypic models actually imply wheat is 10 times more complex than us. While this conclusion could possibly be true in some very limited domains I again stress that this is so contrary to what we would expect that it indicates genotypic models are not the most suitable way of measuring complexity for our purposes. The problems caused by the developmental environment and the problems of a genes indirect and pleiotropic impact support this conclusion about the (un)suitability of genotypic models. The Morphological Midpoint I will now consider one “intermediate position” in the spectrum of models which measure complexity. Such models include those which don’t use only the genome but are still based on heterogeneity or information theory. Unfortunately, due to space, I will not consider this kind of model here (although I think they all fall prey to the one objection, which is that the kind of complexity we are interested in is not the same as the length of the description). Instead, I will consider a model put forward by McShea and Changizi (2003), and others, which is aimed at addressing the extent to which a species is morphologically complex. McShea (2000) contended that morphological complexity correlates sufficiently with behavioural and functional complexity that to try and include more than morphological complexity is to over count. Note that the model developed by McShea and Changizi and others could be applied to any of a number of systems. It is (to borrow a term from philosophy of mind) substrate neutral, and indeed when developing a fuller system I will use McShea’s model several times. I will now first explain what this system is, and then identify three reasons the morphologically constrained version suggested by McShea (2000) is insufficient. McShea and Changizi’s model (2003, p74-75) seeks to measure morphological complexity (remembering that McShea argues this implies behavioural and all other forms of complexity) in a hierarchical, two tiered way, with the first tier based on nesting and the second on individuation. The first tier is the level of nestedness, i.e. the number of times the second level tiers of individuation have nested. These tiers are numbered – so if an organism is made up of no lower level tiers then its degree of nestedness would be “1”. On the other hand, if it were made up lower level parts which were in turn made up of yet lower level parts it would be described as having a nestedness of “3”. The minimal level of nesting is (to an extent) arbitrary. This arbitrariness is acknowledged (ibid, p75) when Prokaryotes are placed at level 1, although McShea and Changizi say a level must be a level of complexity at -4- which selection has taken or takes place (ibid, p75)4. This approach to placement is generally in line with our intuitions and will be retained in this essay. The lower, fundamental tier (which drives the model and gives the context used to determine which top-level tier an organism belongs to) measures an organism’s complexity by the number of kinds of parts it has and the kinds of relationships between these parts, i.e. the level of individuation in an organism’s components. In this second tier an organism can be at one of three discrete levels of complexity (a, b or c). It may just be an unstructured collection of several lower level parts, where “parts” are one-tier-down things – that is, “a set of [presumably lower-level] components that are relatively well connected to each other and relatively well isolated from other components” (McShea, Anderson 2001, quoting McShea, Venit, 2001). An unstructured aggregation of undifferentiated parts is called sub-level a. For example, when a cellular slime mold swarms, it is just a collection of (lower level) individual cells with no structure or differentiation amongst the types of cells and it is therefore at sub-level a. Based on the fact that it is just composed of eukaryotic cells which are level 2c (historically, eukaryotes are the result of two or more prokaryotes merging) the overall complexity of a swarming cellular slime mold is probably 3a. However if an organism is composed of two or more different kinds of less nested parts which are still in an unstructured aggregate McShea places this organism at level b. The distinction between a and b strikes me as a justifiable one as there is an important difference in the apparent level of complexity of something which is made up of just one kind of thing and something which is made up of two or more. For example, slime mold reproduces through spores or cellular fission, i.e. through the behaviour of one type of cell. Vaucheria, a genus of alga used by McShea as an example of level 3b, is different though. Vaucheria can be comprised of several kinds of cell, including both sperm and eggs. The physical structure and the repertoire of behaviour and interactions amongst these multiple types of cell is significantly more complex than the behaviour which takes place in slime mold. At sub-level c each organism is a single structured (hierarchical) collection of two or more types of lower level parts. This is obviously more complex than an unstructured clump of different things (sub-level b). An example of this also used by McShea is coral. Any one (hermatypic scleractinian) coral is in fact a morphologically structured symbiosis of zooxanthellae and the cells of the coral itself (Levinton, 1982), and this is presumably why coral is placed at level c (McShea does not say and I am not certain). If an organism were more than one structured collection of lower level parts it would be a member of a higher primary (nesting) group, with a sub-level of either a or b. For example, if it were an unstructured aggregation of its structured parts it would be level a. While McShea and Changizi (2003, p79) attempt to apply this schema to relationships between intentional (humans) or at least semi-intentional (honey bees) species they acknowledge that this very morphologically based model is “difficult to evaluate objectively”. I will now examine three of the other problems such a morphologically focussed model of complexity has in more detail. 4 Which immediately prompts the query: surely selection operated at the pre-prokaryotic level of complexity as well? -5- The first of these problems regards the crucial assumption made by McShea and Changizi that morphology and behaviour have a predictable many-to-one or one-to-one relationship. While this assumption may well be true for less complex species the complex interactions between a primate’s perceptual systems, its brain and the physical, social and biological environment5 show how this relationship becomes a many-to-many relationship and that McShea and Changizi’s model must therefore have limited applicability if behaviour is not somehow incorporated. Of course, incorporating behaviour in an ad hoc manner will almost certainly simply result in the same kinds of problems faced by genotypic models which try to incorporate the developmental environment. Secondly, the general definition of complexity in use and the target of this essay must undeniably include more than the morphology – for example, few would dispute the claim that, overall, the eusocial honey bee is more complex than the mud-daubing wasp Trypoxylon politum. While a purely morphological definition of complexity may be sufficient in other circumstances this means it is not sufficient in helping us understand what we mean more generally. Thirdly and finally, as Sterelny and Griffiths note (1999, p370): The central empirical idea defining emergence is that surprisingly complex system-level behaviour can arise out of locally interacting simple units. Complex behaving systems require neither complex parts nor central direction. The elements in A-life models are often quite simple units whose interactions are all governed by local rules – indeed, relatively simple local rules. But the behaviour of the system as a whole is often adaptively complex. Some social insect colonies may provide natural examples of the phenomenon in question. Simply interacting simple creatures nonetheless produce complex, adaptive, and patterned behaviour. So a good many of the more striking examples of the A-life models can be seen as undercutting the idea that fancy systems must be built of fancy components. They show that complex system-level behaviour may arise out of interacting simple components. In other words, complexity can arise emergently from morphological simplicity. For these three reasons, and also from extensions of many of the problems genotypic models of complexity face, I think we can conclude that a purely morphological model of complexity will not be wholly sufficient for our purposes without becoming overly and dangerously ad hoc. The Hydra of Complexity Hence, we move on now to the fourth part of this essay and the broadest end of the definitional spectrum. In particular in this section I would like to make sure the different dimensions along which a species may be complex can be recognised. This is important (as the examples used below show) because complexity or simplicity in one area, such as morphologically or genetically, does not necessarily translate into complexity or simplicity elsewhere. A broad definition of a species may mention a range of high level elements, such as behaviour and morphology. Both of these aspects will be incorporated The physical environment is the static environment – rocks, trees, rain and so forth. The social environment is the other organisms with which an organism interacts with on a regular basis. The biological environment is every other living entity with which an organism interacts. 5 -6- into the final model I will develop. A shopping list of possible elements will be developed and then selected from as the model is developed. How the complexity (for each element) of two species can be compared will be decided on following each selection. Prior to enumerating some of the members of this list though I think it is important to highlight that at this end of the spectrum there are a number of ways of decomposing complexity into its components. If too broad divisions are used – for example morphological complexity and behavioural complexity – then a comparison of the complexity of organisms from different species remains subjective. Is the behaviour of the ant lion more, less or as complex as the behaviour of the mud-daubing wasp Trypoxylon politum? As Kim Sterelny suggested, if the organisms in question were from very similar species then some comparison could still be made, however I am seeking to develop as general a framework as possible, and this means that the divisions have to be carefully precise. On the other hand, too many higher level factors may reintroduce the problems which occurred with genes, where multiple apparently independent components are actually redescriptions of the same thing. If the range of habitats an organism can live in is considered one measure of its complexity, how is over-counting avoided if the number of life cycle stages or modes an organism has is another? It may seem that a bear which hibernates as well as living through a hot summer is as or nearly as complex as a liver fluke (Dicrocoelium dendriticum), whose life cycle stages are described by Dawkins (1982, p218). In summary, careless use of conceptual, subjective measures of complexity is likely to lead to confusing results which disagree with our intuitions too frequently. While we shouldn’t expect our intuitions to be confirmed all of the time, at some point we need to make a meta-judgement about a decision making system itself and decide that it is leading us astray. I conclude that a careless, too-conceptual approach is likely to do this, due to incompleteness, subtle over-counting or excessively subjective divisions. These are potential problems which will be mentioned again as the high-level, multidimensional (broad) model we need is created. Bearing in mind the danger of being either too vague or too precise I have divided the measures of a species’ complexity into four components – a species’ morphological, perceptual, behavioural and environmental complexity. These components broadly seem to cover all of the ways in which a species may be complex, i.e. they are complete. A species may: Have a physically complex structure or life cycle. Perceive the world in a complex manner. Behave complexly. Or have a wide ranging and high fidelity impact on the environment and on other species (colloquially, its “environmental complexity”). At first glance, these divisions seem to be quite independent. This is because an organism could be complex in any of these ways, but not necessarily in another. For example, the activities of the ant species Formica aquilonia, which “farms” aphids and builds large ant colonies, indicates it is (environmentally) relatively complex. Morphologically though -7- any single ant – or even a colony of ants somehow summed – is still a little bit simpler in comparison to other species. If an ant were compared to a cheetah it would be fairly clear that the cheetah had a slightly more complex morphology. Despite this morphological complexity though, most predators – including cheetahs – have a much simpler eat/fleet/ignore relationship with other species and with the physical world. Either they eat another thing, they flee from it or they ignore it. Farming is a much more subtle and complex task. However, while it is possible for the divisions to be independent it is also quite easy for them to overlap. When viewed from such an abstract level, an organism’s environmental complexity is, in many ways, just another way of describing its behavioural complexity. There are important differences, in that the consequences of behaviour with a more significant environmental impact may make the organism intuitively seem (and thus, for our purposes, be) more complex than similar behaviour without such an impact would, but these overlaps do exist and must be addressed. They would frequently occur because the four divisions are only loosely defined and thus seem to have a significant degree of causal connection between them. For example, a complex perceptual system and physical morphology is often (but not always) needed for complex behaviour6, just as complex behaviour is often (but, again, not always7) needed for the members of a species to have greater environmental complexity. One way of addressing these overlaps would be to revisit the four fundamental divisions I outlined above. Unfortunately, careful thought has not helped me create another set of divisions which is as complete, as useful8, and which is more independent (rather than just being as independent in a different way). Another approach to addressing this independence problem is to try and further decompose the four groupings outlined above. By doing this the locations of any overlaps will become clearer and their impacts can be minimised by selecting items from the resulting “shopping list” in ways which mean they overlap as little as possible. I have pursued this approach and detail it below. Physical and Temporal Complexity I would argue that the morphological elements of an organism’s complexity can be broken down into spatial and temporal complexity (i.e. life cycle stages). I suggest using an organism’s physical, structural, hierarchical complexity (how this can be measured is described in (McShea, Changizi, 2003) and above)9 as a measure of its spatial complexity. Some useful elements of the concept of heterogeneity are captured by this model. In addition to McShea’s hierarchical model it could be suggested that there is some limited place for the complexity of the developmental environment. I am suspicious of such an approach though due to the danger of over-counting – almost all developmental 6 It was for these reasons that McShea (2000) saw morphological complexity as a ubiquitous stand in for other forms. 7 If it were “always” we could simply draw a causal flow and measure complexity from the beginning of this causal flow, since all future complexity must necessarily be entailed in the earlier steps. In other words, we would be right to use McShea’s (2000) model. 8 I claim the above four divisions are particularly useful because they provide a logical way of decomposing the aspects of a species which contribute to its complexity. 9 While their model alone is insufficient as a way of measuring all complexity it does assess morphological complexity well. -8- complexity will be reflected in the hierarchical complexity (whereas it is not in the size of the genome). While the inclusion of developmental complexity might make the way in which complexity is measured slightly more complete it would also make it significantly less independent and therefore it is not included in this system. Simply counting the number of life cycle stages gives the most important and accurate measure of temporal complexity. While these could be reasonably blurry “stages”, such as occur in a human as they grow from (birthed) child to adult I think that a strong case could be made for such a process to be almost inevitable and no significant contributor to complexity. Instead, I would restrict this measure of complexity to a “higher level” in the hierarchy of life cycle stages, of the kind seen in butterflies and caterpillars – changes significant enough that we almost have to give the folk organism a different name, simply to avoid confusing ourselves. Perceptual Complexity Perceptual complexity is analogous to a pipe. A pipe’s “complexity” can be measured by its diameter and by the speed water flows down it. Similarly, perceptual complexity can be measured by the number of ways a species can perceive the environment (its phenomenological breadth) and also by how precisely it perceives its environment (its phenomenological fidelity). In addition to the breadth most other insect species possess, a cockroach has a low fidelity pressure detector – when it senses any increase in air pressure it will move as quickly as possible away from this pressure increase. This pressure increase may be only a passing wind – but it may also be a descending hand. Thus cockroaches have greater breadth and fractionally greater fidelity than a stereotypical “base” insect. Similarly while a human and a dog may have very similar or identical phenomenological breadth (both species can see, hear, smell, taste and touch) a human has greater visual fidelity, while the dog has greater auditory and olfactory fidelity. Note here that perceptual and morphological complexity do not significantly overlap (in the way they are being used to measure complexity) – morphological complexity is completely afunctional, while perceptual complexity is purely functional and reflects an organism’s ability to receive information from the world. It could be claimed that broad or high fidelity perceptual systems always require spatial complexity, but I think the hierarchically equal spatial complexity of a starfish (e.g. Asterias forbesi) with a species that has clearly greater perceptual complexity (e.g. Canis lupus familiaris) makes such a case unlikely. Note that I say that a starfish and a dog are spatially equally complex because a starfish, like a dog, is internally differentiated into a range of organs with specific functions. Each organ is in turn composed of several types of cell. Breadth is accurately measured at a gross level by simply counting the number of ways in which an organism can perceive – thus for humans you could note the proprioceptive, visual, auditory, tactile, olfactory and gustatory components of our perceptual abilities. It may be argued that this kind of division is too gross though and that some elements of an organism’s phenomenological breadth (such as visual) deserve greater weighting than others (such as gustatory) or that these elements should be treated as more than one system. Very briefly, I have a two part response to this objection. Firstly I would note that suggesting that one perceptual system (such as vision) is in fact more than one -9- system starts to drift towards evaluating the fidelity of the system. The issue of how to evaluate an organism’s phenomenological fidelity will be considered very shortly. Secondly, I am cautious here of anthropomorphic bias. Perhaps vision seems like it is more than one perceptual system to us because our visual systems are so rich and well developed, but a dog (or an ant) may have a very different opinion. Fidelity is a less grounded measure and unfortunately no species-independent approach can be given. It would be a mistake, for example, to assume that humans perceive the world in a particularly perfect or accurate way. Many psychological experiments (e.g. optical illusions which lead to conscious and explicitly contradictory perceptions such as colour phi10, and experiments which manipulate our memory of our perceptions, as discussed in Dennett, 1991, p116, p117, p120, p123) have shown we do not. This unfortunately makes comparison of, say, visual fidelity with olfactory fidelity very difficult and I do not have the space to do more than flag the existence of this problem in this essay. This lack of an objective grounding or “zero point” and the frequent incomparability of two different ways means that we can really only assess one species’ phenomenological fidelity in comparison to another’s and not against some objective measure. There will also be many situations where the fidelities are so similar, or our knowledge is so lacking, that comparing the two organisms in question to each other directly is very difficult. However, while a scalar and continuous ranking (“this one is 3.23 times better than that one”) is not possible we may still be able to order them in the same way a lot of geological and paleontological data is ordered. This is done through reasoning that, for example, since this sediment was laid on top of this one it must be younger. At a different location, since it is underneath a third, the third must be younger still. In geology and paleontology though there is access to objective grounding measures such as carbon-14 dating (Knoll, 2003, p54). These let an ordering be turned into something semi-scalar and continuous in some situations through comparison with well known reference points (Knoll, 2003). For example: “this rock is older than that rock, which is 13.5 million years old”. This is not possible for phenomenological fidelity. It might seem that one method of addressing this problem with phenomenological fidelity, a method which also allows clearer comparisons of different ways of achieving phenomenological breadth, is to measure an organism’s perceptual complexity at the information theoretic level (i.e. how many binary bits of information the organism can perceive per second). This may seem like a particularly valuable and speciesindependent approach if we consider some of the difficulties raised above in comparing (for example) a human’s and an ant’s perceptual complexity. While I will discuss the problem of comparison in the multi-dimensional system I am in the process of building more generally later I’ll consider this suggestion here while the discussion of perceptual complexity is still fresh. This information theoretic measurement would span both breadth and fidelity but implementing it requires that we address a number of pragmatic problems. Developing different systems for each of the different ways of perceiving which accurately and objectively measured the quantity of information perceived would be difficult. Exactly how many bits per second does an ant olfactorily receive in a pheromonal environment? We could quite easily establish some lower bound, but this would almost certainly under 10 The dots are both moving/changing colour and not moving/not changing colour. - 10 - estimate how much information it did actually perceive, just as measuring how fast a human can type or otherwise output information almost certainly underestimates how fast a human can think. Even doing this in a perceptual system-dependent and somewhat subjective way though (which would mean the objective comparisons we adopted an information-theoretic system to do couldn’t be done) would be very difficult: how would we go about determining how many bits of information a human perceived visually in one second? And how would we go about doing that for a honey bee? Trading one form of subjectivity for another more confusing one seems like a poor deal to me. In addition, it is quite possible that we may perceive at different bit rates at different (conscious) times. If I were personally focussing intently on my proprioception while learning to do something gymnastic, nearly to the elimination of everything else such as what I could perceive visually, and if my proprioception is of lower bandwidth than my visual system (and I think it probably is) then my perceptual complexity suddenly got a lot less and became very contingent. This thus poses the question: which bandwidth at what time is an organism’s true phenomenological breadth and fidelity? For these reasons I am very wary of an attempt to build an objective and grounded measure of perceptual complexity, but I draw the discussion of perceptual complexity to a close here for now. Behavioural Complexity Bearing particularly in mind how behavioural complexity overlaps environmental complexity I suggest that an organism’s behaviour can be broken down into (as with morphological complexity) two key sub groups. The first of these is its flexile complexity – its flexibility and responsiveness to its environment, an area of overlap with any epistemic engineering an organism does (which is one potential contributor to its environmental complexity11). Incorporated into this flexibility and responsiveness is of course the range of behaviours which can be exhibited and also the ability to learn. Directly measuring the complexity of this behaviour is difficult. This could indicate that: Further decomposition is necessary. It is an inherently subjective thing, and therefore this is a bad decomposition. Flexile complexity is simply difficult to measure. Without eliminating the third possibility as the cause of the problem neither of the first two should be considered. As it turns out it was the third possibility which led to the difficulty and it could be resolved by approaching the measurement of flexile complexity from a different perspective. I suggest that a species’ level of flexile complexity be compared with another’s by comparing the order12 of the number of possible pieces of environmental information (specific noises, visual patterns, sounds, odours etc) an organism can use in selecting an action to perform. Thus a dog (Canis lupus familiaris) is one or more orders more flexibly complex than a cat (Felis silvestris catus), as the dog can respond to a greater order of audible cues. The dog can behave differently depending 11 As I judge that the behavioural perspective is more complete than that offered by the environmental elements of it (namely, the epistemic engineering) I will eliminate this overlap by not considering epistemic engineering as a part of environmental complexity. 12 “Order” is currently loosely defined, but is some combination of the definitions of the cardinality (of an infinite set) and order of magnitude. Space constrains a more detailed definition. - 11 - on what seems like (based on historical results from training dogs) an effectively infinite number of possible noises. This constitutes a higher order of flexibility. The second type of behaviour which contributes to behavioural complexity is how an organism interacts with others from its own species – interactions with other species I’ve placed under environmental complexity, to limit overlap. This form of complexity will be measured by the extent to which one organism can communicate with another. Other aspects, such as its ability to cooperate, are a consequence of communicative ability. It can be broadly measured by division into discrete degrees13. The first of these is the absence of almost all communication between members of the species. This occurs, least interestingly, in viruses and any bacteria which signal in simple ways, and may occur in some morphologically more complex species as well, such as the fruit fly Drosophilia melanogaster. The only communication that does occur within such species is what we might optimistically call “ritualised courting”. At the next level I would place most pack animals and perhaps the eusocial insects which signal relatively simple information to others through the use of pheromones and so forth. The nature of the communication that takes place within a lion pride may be rougher and of slightly lower fidelity than that which takes place in an ant colony, but it is clearly of a higher degree than that which occurs amongst most bacterium and is relatively close to that which occurs in an ant colony. Similarly, both ant and lion communication is clearly of a lower degree than that which is known to occur amongst humans, other eusocial insects and perhaps some primates (other primates are in the second level of communicative complexity). In this kind of communication, the third and final level, the information which is communicated is again structured in a more complex way. Instead of its semantic content being immediately present the communication can refer to (for example) possible events, events which happened in the past, objects out of sight of one or more of the parties in the communication (e.g. honey bee flower location dances) or abstract and non-physical entities. Tangenting briefly, it has been observed that the discrete degrees selected could just be elements plucked from a continuum. I have no strong opinion on what the underlying nature of communicative complexity actually is though, and I don’t think I need to. While it may be the case that it is a continuum it also seems to me that the three degrees selected at least form the centres of quite significant “clusters” of many organisms’ levels of communicative complexity. If we conclude that there is in fact a continuum here this will not change the fact that most communication appears to clump around the three degrees discussed above and that they provide useful initial starting points in measuring complexity. Relative rankings can be done (as they were for lions and the simpler eusocial insects, above) as needed and as described in more detail for phenomenological fidelity. As with McShea’s hierarchical approaches and flexile complexity comparing within a degree is very difficult – in large part I claim because this is an area which is potentially dangerously subjective and anthropomorphic. In addition, the ability to objectively ground a measurement of an organism’s complexity has been lost, as it was for perceptual complexity. I will discuss this point in more detail later. 13 - 12 - Assessing whether or not a species actually truly belongs to the third level or is instead a complex member of the second, and whether a fourth (or fifth or…) level exists are thorny questions. The former can probably be addressed by very carefully structured psychological experiments which aim to be as anthropomorphically distant as possible. The theoretical possibility or impossibility of a fourth-plus level (the second question) may be answerable with deep thought or it may be fundamentally beyond the cognitive capacities of the human brain to answer. In any case, we possess the ability to judge into which level we think each species falls, and it is unlikely any species will be in the elusive fourth-plus level. If it were it would surely obviously fit in the third level and it would probably also (in some way) seem smarter or a better cooperator than us14. Environmental Complexity Environmental complexity is the most difficult form of complexity to measure in a species-neutral way. There is a significant degree of risk that any conclusions we draw will be anthropomorphically biased (Rivas & Burghardt, 2002). Thus, moving carefully, I think the initial decomposition regarding an organism’s environmental complexity is to break it into the two fundamentally different elements which comprise it – complexity with respect to the way it interacts with the environment and with members of other species which don’t react back (e.g. birds and most trees), and complexity with respect to the way it interacts with other species which do react (static complexity and interactive complexity, respectively). Interactive complexity, as with communicative complexity, is divisible into three simple degrees. The simplest way in which a member of one species can react to a member of another is precisely that – to simply react. It may eat it or flee from it or (as will be the case for the vast majority of species) simply ignore it. The most complex way members of different species have been known to interact is through prolonged symbiosis. Ant (and human) farming of aphids (and cattle, respectively) are examples of this kind of symbiosis. In this situation each species, to some extent, specialises further and members of the two species mutually exploit. Does this prolonged symbiosis necessarily contribute equally to the interactive complexity of both of the symbionts? No – if one can survive without the other then the “symbiosis” is more like exploitation in which it benefits the exploiter to keep the exploitee in an optimal state for at least some period of time. Human exploitation of cattle is definitely an example of this. On the other hand maybe one (or both) of the species has its fitness partially but not absolutely reduced without the other. The symbiosis/exploitation relationship is thus a continuum and it is along this continuum that within-degree comparisons could be made. It is possible that the exploitee may actually be at the fight/flee degree. Such a situation would have to be evaluated on a case-by-case basis and very carefully, so as not to bow to human anthropomorphism. Intriguingly – and not a point I’m going to investigate in this essay – does this mean that we can’t rule out eusocial insects and other super-organisms as communicating at the fourth level? Do you have to communicate frequently at the n-1’th level to be judged to communicate at the n’th? Although flower dances are a form of 3rd degree communication they happen with much less frequency than 3 rd degree human communication (“what are you going to do today”, “where have you been”, etc ad infinitum) – just as humans exhibit 1st and 2nd degree communication much less frequently. 14 - 13 - The second level is the mid-point of these two levels – namely sporadic symbiosis in which there is no prolonged relationship. Such a situation occurs with an Egyptian Plover bird and a crocodile. A particular crocodile or Plover bird, or group of crocodiles or group of Plover birds, do not in any way “team up” over a prolonged period of time. A crocodile and Plover will only interact a second time if they happen to remain in the same spatial area. This is hardly sufficient for the relationship to constitute prolonged symbiosis, rather than repeated one-off symbiosis! Finally, it might seem that fight/flee/ignore should actually be fight/flee and ignore – that is, separate levels, with the lowest being for an organism to not react at all to a member of a different species. However this is likely due to perceptual simplicity or the irrelevance of members of that species to the fitness of the organism whose complexity we are evaluating. In the former case the complexity (or lack thereof) will have already been taken into account and in the latter it has no bearing on an organism’s interactive complexity. Static complexity is the extent to which a species engineers its environment. Excluding epistemic engineering (to avoid over counting with flexile complexity) a species niche constructs (Sterelny, 2003, p1) to achieve one or more of four goals – to help it catch prey (a spider web), to help it avoid predators (Dawkins, 1982, p200), to improve its reproductive fitness (as “dummy nest” building birds do) or to aid in resource gathering and protection (a bee hive). However if we were to try and measure complexity by measuring each of these we would create an over complicated model which overlapped with itself to too great an extent. Therefore we need to look deeper, for some common element to all of these tasks. A range of possible approaches to this problem exist. Some of the immediately obvious ones include the size of the structures constructed relative to the size of the organism, the number of generations the structure persists, the number of organisms which use a specific structure and how much time is invested in either creating or using the structure. We can dismiss the idea of using relative size though, as it seems to me that human artifacts (such as microprocessors and other electronic equipment) show that there is not necessarily a positive relationship between size and complexity. Similarly, larger objects are often more complex than smaller ones (compare an ant colony to the hole dug by a mud daubing wasp!). This means there is not a consistent negative relationship either. Hence, using relative size will give inaccurate results. For similar reasons and using the same or similar counter-examples we can dismiss measurements based on the number of generations the structure persists for, the number of organisms which use it and the time invested in creating it. Human mass manufacturing must surely immediately show the latter to be particularly problematic! Thus, as with spatial complexity, I think we need to examine the degree of nesting and individuation of the structures engineered by a particular species. Implicit in this model (as with spatial complexity) is the assumption that complexity exists for a reason, i.e. it is either adaptive or is an adaptation. An example of an application of this approach is that of bird nests. At the simplest level of niche construction amongst birds we find scrape nests, created by a range of species, which are depressions scraped (or found) in the ground, perhaps with a few stones added haphazardly (Ritchison, 2005). Such nests are at the lowest level of complexity (1a): they - 14 - are composed in an unstructured way of undifferentiated parts which are themselves not structured hierarchically. On the other hand the nests of the small passerine (Sterelny, 2003, p3) and Horned Lark (Eremophila alpestris) (Ritchison, 2005) are much more highly structured. While the parts they are composed of are still themselves lowest level parts they are often of several types, carefully selected and specifically arranged, to camouflage the nest against predators or to shelter it from the prevailing wind. These nests would be level 1c. Human habitation, on the other hand, is composed of at least two or three levels of nested parts: There are several types of room, which maybe could be considered to be organised in a structured hierarchy. Each room is of course composed of the structural elements and the functional furniture, each of which is also composed of a nested hierarchy of parts: a wall is made up of beams and plaster and maybe paint or wall paper. A beam is wood, but it is also (deliberately) made of rivets and other joiners – thus human habitation seems to be level 3b or 3c. Of course, this model allows potentially incorrect conclusions to be drawn as well – for example, that some specific members of a species are more complex than other members of that species. However this problem can only occur if environmental and circumstantial contingencies are ignored. To say that a specific Horned Lark is less complex than another because there is insufficient construction material available for it to build a shield against the wind is a weak argument. In addition, another problem may appear to exist with this approach to measuring static complexity. Static complexity and the communicative complexity appear to causally bracket task complexity (Anderson, Franks, McShea, 2001). By this I mean that task complexity is entailed by communicative complexity, and it might appear that static complexity is entailed by task complexity. This is particularly so since much niche construction may be done in groups or teams (crucial to task complexity). If task complexity is entailed why isn’t static complexity? Before explaining why task complexity does not entail static complexity though I think it is important to first describe task complexity so that McShea, Franks and Anderson’s paper (2001) does not need to be immediately referred to. Task complexity is a method for measuring “the degree of cooperation and coordination required for successful task completion, based upon the deconstruction of a task into its component tasks and subtasks” (ibid, p644, quoting Anderson, Franks, 2001). Tasks are divided up into three types. They can either or only be completed individually. This is the simplest level of task complexity. Alternatively, they may be group tasks. In a group task all individuals carry out the same process, “crucially though, individuals must work concurrently or the task cannot be completed” (Anderson, Franks, McShea, 2001, p644). This is typically because there is some threshold or tipping point (even a literal one) before which the efforts of the individuals have no effect. The food item (for example) may simply be unmovable by one individual alone. It is noted that parallel work (where each individual carries out the same task, such as all feeding a different larva at the same time) does require coordination but that it does not qualify as a group task because any one individual can still carry their instance of that task to completion by themselves. Thirdly and finally, tasks may be team tasks. Such a task requires that two or more different sub-tasks are carried out simultaneously in order for the overall task to be achieved. Alternatively (and judged to be just as complex) the tasks may need to be - 15 - carried out sequentially. An example of such a partitioned task is the collection, transport and processing of some resource. I will now explain why the causal flow outlined above (communicative → task → static) might be suggested, but also why it breaks down and specifically why it breaks down between task and static complexity but not between communicative and task complexity. The reason for this is relatively simple. While it is undeniable that task complexity requires communicative complexity – you cannot have a significant degree of team work without communication of some kind! – and is therefore entailed by it, it is also undeniable that you do not need communication in order to have a complex impact on the physical environment. After all, many organisms can create complex static structures quite successfully individually, even if they do communicate with others some of the time. Bird nests are an ideal example of this – if there is any teamwork then each member of the team carries out the same tasks as the other members, meaning bird nest construction has the lowest possible task complexity. Intuitively though the nest of a Horned Lark seems much more complex than that of a sea gull, even though only one lark is involved in the construction of its nest (Bent, 1942 and Sutton, Parmalee 1955). Therefore, to create an as complete, accurate and independent measure of complexity as possible we need to appear to bracket task complexity in this manner. Conclusion Let’s summarise the state of play and any problems this system has. A system which can measure a species complexity across eight dimensions has been built. These eight factors cover the intuitive definition of complexity in a fairly complete and independent manner. Morphological (spatial and temporal) complexity is accounted for, as is the wider functional impact in terms of perceptual (breadth and fidelity) complexity. Behavioural complexity at the individual or species level (flexile and communicative) and at the wider environmental (static or interactive) can also be measured using this system. Actual measurements for any one factor are carried out using one of four techniques. Spatial and static complexity can be measured using the McShea/Changizi hierarchical approach. Communicative and interactive complexity are measured in degrees, and temporal complexity and phenomenological breadth are simply counted. Flexile complexity and phenomenological fidelity are measured in orders. Measurement using any one of these techniques is relatively easy and is as objective as possible. There are two key problems this system has not addressed though. The first of these is a result of the inherent subjectivity in the term complexity, and it is a lack of what I term “grounding” for many of the factors, discussed briefly already in the context of phenomenological fidelity. Grounding is simply the process of attaching a unit and zero or minimum level to a measuring system. When this is done measurements in that system are scalar and are also probably continuous. For example, because mass is measured using a scalar system in which the concept of 1 and 0 grams is meaningful, we can say that one kilogram is twice as heavy as 500 grams. In addition mass is continuous (at least to the level of sub-atomic particles): between any two levels of mass you can always find another, intermediate level of mass. Low level physical phenomena like mass are relatively easily grounded, at least at the macroscopic level. Any grounding of a system which measures complexity would be arbitrary though. This leads me to question the utility of being able to say “this species is three complexiles simpler (given our arbitrary - 16 - definition of one complexile and zero complexiles)” and consequently the significance of this problem. The second is a consequence of seeking as much independence between elements as possible. Because of this we cannot say that one species is more complex, overall, than another. While we certainly can trade off and compare complexity in different dimensions each such trade off will have to be done on a case by case basis. Ensuring that these trade-offs retained species-neutrality might be quite difficult. In any case, this is not the focus of this essay. As a result, when using the system as it has been described here it is simply not possible to say that a liver fluke is more complex than a honey bee. However, this negative aspect is not particular to this system, as it is an inherent problem in our general definition of complexity and therefore in any attempt to systemise it. With this general definition we clearly feel we can say one organism is absolutely more or less complex than another, and we also feel that there are multiple independent elements which contribute to this complexity orthogonally. These two positions are simply not logically reconcilable – no matter how hard you try, you cannot translate a two dimensional space into an equally expressive one dimensional space without making some dramatic assumptions, assumptions which tend to invalidate the translation15. Given this conundrum I have elected to develop a system which measured complexity as completely as possible, at the cost of some simplicity. Wimsatt’s discussion of complexity (1972) in fact suggests that a meaningful overall comparison cannot be made. Excluding the trivial and rare case where one species is more complex than another on every dimension he may well be right (although this question cannot be investigated in this essay). With that I will draw this essay to a close. A few contortions have given us a system that is as species-neutral as possible. Provided we compare apples with apples we can make meaningful comparisons of organisms’ complexity. The danger of over-counting has been examined, and the difficulty of measuring complexity at a very fine level has been discussed16. The importance of completeness and independence (or orthogonal dimensions) in the type of system we have developed has also been stressed. 15 Just try finding some way of translating all two dimensional positions in some plane into positions (which retain their relative magnitudes and positions) on a one dimensional line! 16 Some of the “apples” want to compare could be made more fine grained. This is clearly outside the scope of this essay, although some possibilities were hinted at, e.g. in interactive complexity. - 17 -