Supplemental data
advertisement

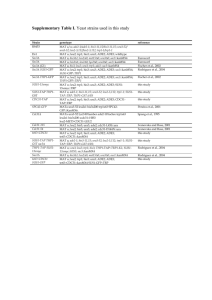
SUPPLEMENTAL DATA The Rad52 homologues Rad22 and Rti1 of Schizosaccharomyces pombe are not essential for meiotic interhomologue recombination, but are required for meiotic intrachromosomal recombination and mating-type related DNA repair. Guillaume Octobre*, Alexander Lorenz†‡, Josef Loidl† and Jürg Kohli*§. *Institute of Cell Biology, University of Bern, Baltzerstrasse 4, CH-3012 Bern, Switzerland, and †Department of Chromosome Biology, University of Vienna, Dr Bohr-Gasse 1, A-1030 Vienna, Austria. ‡ Current address: Department of Biochemistry, University of Oxford, South Parks Road, Oxford OX1 3QU, United Kingdom. § Corresponding author. Mailing address: Institute of Cell Biology, University of Bern, Baltzerstrasse 4, CH-3012 Bern, Switzerland. Phone: +41 31 631 46154. Fax: +41 31 631 4616. E-mail: juerg.kohli@izb.unibe.ch -1- SUPPLEMENTAL MATERIALS AND METHODS Spot assays for genotoxin sensitivity: The strains to be assayed were grown to an OD600 of ~0,8 (about 107 cells/ml). The cultures were then serially diluted (10-fold steps) and 10µl of each dilution was spotted on YEA plates supplemented with the indicated amount of genotoxins. For each genotoxin-supplemented plate, an unsupplemented YEA control plate was prepared. The plates were incubated at 30° for 3 days, and then photographed. Determination of mating efficiency: To determine the mating efficiency of crosses, the number of cells (C), zygotes (Z), asci (A) and spores (S) in a sample of the crossing material (see “Material and Methods”) were counted by microscopy. The mating efficiency was calculated by the following formula: (A+Z+0.25S)/(A+Z+0.25S+0.5C) x 100% (GRISHCHUK et al. 2004). A minimum of 500 cells were counted in each experiment. Protein extraction and immunoblotting: Protein extraction was done by trichloroacetic acid (TCA) precipitation as described previously (FOIANI et al. 1994). For Western blotting, proteins were separated by electrophoresis on 10% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane (Immobilon-P, Millipore). After overnight incubation in blocking buffer (PBS + 5% nonfat dry milk), rabbit polyclonal anti-Rad22 or anti-Rti1 antibodies (described in (VAN DEN BOSCH et al. 2002)) were applied at 1:5000 dilution for at least 1 h at room temperature. The membrane was then washed and incubated at room temperature with an adequate HRP-conjugated secondary antibody for at least 1 h. The antibody-bound proteins were detected using the ECL plus Western blotting Detection system (Amersham Biosciences, USA), and pictures were taken using a LAS-1000 luminescent image analyser (FUJI, Japan). -2- Mitotic whole cell immunostaining experiments: Proteins were localized in paraformaldehyde-fixed cells following the protocol of (ALFA et al. 1993), except that cell walls were digested with 0.6 mg/ml Zymolase 100-T (Seikagaku, Japan) for 70 min at 37°. Polyclonal anti-Rad22 and anti-Rti1 antibodies described above were used at dilutions of 1:500, together with appropriate TRITC-conjugated anti-rabbit secondary antibody. After staining, cells were mounted in Vectashield mounting medium (Vector Labs, USA) and examined using a Nikon Eclipse E600 epifluorescence microscope equipped with an Osram mercury lamp 103W/2. Pictures were taken using a Nikon DXM1200 polychrome digital camera. -3- LITERATURE CITED ALFA, C., P. FANTES, J. HYAMS, M. MCLEOD and E. WARBRICK, 1993 Experiments with Fission Yeast. A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. FOIANI, M., F. MARINI, D. GAMBA, G. LUCCHINI and P. PLEVANI, 1994 The B subunit of the DNA polymerase alpha-primase complex in Saccharomyces cerevisiae executes an essential function at the initial stage of DNA replication. Mol Cell Biol 14: 923-933. GRISHCHUK, A. L., R. KRAEHENBUEHL, M. MOLNAR, O. FLECK and J. KOHLI, 2004 Genetic and cytological characterization of the RecA-homologous proteins Rad51 and Dmc1 of Schizosaccharomyces pombe. Curr Genet 44: 317-328. OSMAN, F., J. DIXON, A. R. BARR and M. C. WHITBY, 2005 The F-Box DNA helicase Fbh1 prevents Rhp51-dependent recombination without mediator proteins. Mol Cell Biol 25: 8084-8096. VAN DEN BOSCH, M., K. VREEKEN, J. B. ZONNEVELD, J. A. BRANDSMA, M. LOMBAERTS et al., 2001 Characterization of RAD52 homologs in the fission yeast Schizosaccharomyces pombe. Mutat Res 461: 311-323. VAN DEN BOSCH, M., J. B. ZONNEVELD, K. VREEKEN, F. A. DE VRIES, P. H. LOHMAN et al., 2002 Differential expression and requirements for Schizosaccharomyces pombe RAD52 homologs in DNA repair and recombination. Nucleic Acids Res 30: 13161324. -4- Table A1: List of the strains used in this study. Genotypeb Strain Matinga designation type 1-11 h+ ade1-40 1-26 h- ade7-50 3-87 h- leu2-120 3-99 h- lys4-95 4-150 h+ lys7-2 5-167 h- ade6-M375 6-216 h+ ade6-469 972 h- wt 975 h+ wt 51-2009 h+ ade7-152 EPY17 h+ ura4-D18 ura4A-13 EPY5 h- ura4-D18 ura4A-13 EPY51 h- smt-0 ura4-D18 ura4A-13 FO1471 h+ rad22::kanMX6 ura4-D18 leu1-32 his3-D1 arg3-D4 GO123 h+ rad22::ura4+ ura4-D18 lys4-95 GO124 h+ rad22::ura4+ ura4-D18 lys7-2 GO126 h- rad22::ura4+ ura4-D18 ade1-40 GO127 h+ rad22::ura4+ ura4-D18 leu1-32 ade7-50 GO156 h+ rti1+::3HA-kanMX6 ade6-M210 GO159 h- rti1+::3HA-kanMX6 ade6-M216 -5- GO164 h- rad22::ura4+ ura4-D18 leu2-120 GO165 h+ rad22::ura4+ ura4-D18 ade6-469 GO170 h- rti1::LEU2 leu1-32 ade6-469 GO173 h+ rti1::LEU2 leu1-32 ade6-M375 GO175 h- rti1::LEU2 leu1-32 ade7-152 GO177 h+ rti1::LEU2 leu1-32 ade7-50 GO179 h+ rti1::LEU2 leu1-32 lys4-95 GO181 h- rti1::LEU2 leu1-32 ade1-40 GO184 h- rti1::LEU2 leu1-32 ade6-M210 GO187 h+ rti1::LEU2 leu1-32 ade6-M216 GO211 h- smt-0 rad22::ura4+ ura4-D18 ade7-152 GO214 h- smt-0 rad22::ura4+ ura4-D18 ade6-M375 GO215 h- smt-0 rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 ade7-152 GO218 h- smt-0 rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 ade6-M375 GO228 h- smt-0 rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 ade1-40 GO229 h+ smt-0 rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 ade7-50 GO232 h+ smt-0 rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 ade6-469 GO243 h+ smt-0 rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 lys4-95 GO244 h+ smt0-0 ura4-D18 leu1-32 rec12-Y98F::myc-kanMX6 GO247 h- GO248 h+ GO260 h- smt-0 rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 rec12-Y98F::myckanMX6 smt-0 rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 rec12-Y98F::myckanMX6 rad22::kanMX6 rti1::LEU2 ura4-D18 leu1-32 arg3-D4 -6- a GO267 h- smt-0 rad22::kanMX6 rti1::LEU2 ade6-469::ura4+::ade6-M26 ura4D18 leu1-32 GO271 h- smt-0 rad22::kanMX6 ade6-469::ura4+::ade6-M26 ura4-D18 leu1-32 GO273 h- smt-0 rti1::LEU2 ade6-469::ura4+::ade6-M26 ura4-D18 leu1-32 GO284 h+ smt-0 rad22::kanMX6 rti1::LEU2 ura4-D18 leu1-32 ade6-D20 GO285 h+ smt-0 rad22::kanMX6 ura4-D18 leu1-32 ade6-D20 GO288 h+ smt-0 rti1::LEU2 ura4-D18 leu1-32 ade6-D20 KLY144 h- ade6-M210 ura4-D18 rec12-Y98F::myc-kanMX6 MCW1285 h+ rad22::ura4+ ura4-D18 leu1-32 his3-D1 arg3-D4 MCW1489 h+ fbh1::kanMX6 ura4-D18 leu1-32 his3-D1 arg3-D4 MCW1553 h+ rad22::ura4+ fbh1::kanMX6 ura4-D18 leu1-32 his3-D1 arg3-D4 MCW1686 h+ rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 his3-D1 arg3-D4 MR61 h+ ade6-D20 ura4-D18 MR62 h- ade6-469::ura4+::ade6-M26 ura4-D18 RGL10 h+ rti1::LEU2 ura4-D18 leu1-32 ade6-469 RGL13 h+ rad22::ura4+ rti1::LEU2 ura4-D18 leu1-32 ade6-469 RGL6 h+ rad22::ura4+ ura4-D18 leu1-32 ade6-469 All the GO strains were constructed for this study. The RGL strains were a kind gift from A. Pastink (VAN DEN BOSCH et al. 2001), and the MCW and FO strains from M. C. Whitby (OSMAN et al. 2005). All other strains were taken from the Berne collection. b All the rad22 and rti1 mutants were derived from the MCW1285, MCW1686 and FO1471 strains. -7- Table A2: Mating efficiencies in homozygous crosses of rad22 and/or rti1 single and double mutants. Average mating efficiency (% ± SEM)a Relative average mating efficiency (%)b rad22+ rti1+ (1-11×3-99) 34.7 ± 2.4 100 rad22 (GO123×GO126) 8.2 ± 1.2 23.6 rti1 (GO179×GO181) 30 ± 2.2 86.5 rad22 rti1 (GO228×GO243) 3.7 ± 1.2 10.7 Genotype of the parents and strain designations a The mating efficiency was calculated as indicated in “Supplemental experimental procedures”. The mean and standard error of the mean (SEM) of 3 independent experiments are given. b Values are relative to the rad22+ level, which was set to 100%. -8- Table A3: Spore viability in crosses of rad22 and rti1 single and double mutants. Genotype of the parents and strain designations a Average spore viability (% ± SEM)a Relative average spore viability (%)b Number of independent experiments rad22+ rti1+ (1-11×3-99) 86.9 ± 0.7 100 3 38.5 ± 0.9 44.3 3 76.6 ± 5 88.1 3 rad22 rti1 (GO228×GO243) 3.5 ± 1.1 4 3 rad22 rti1 Rec12-Y98F (GO247×GO248) 14.7 ± 1 16.9 3 Rec12-Y98F (KLY144×GO244) 18.8 ± 0.5 21.6 3 rad22 (GO123×GO126) rti1 (GO179×GO181) a Means and standard error of the means (SEM) are indicated. b Values are relative to the rad22+ level, which was set to 100%. -9- Table A4: Intergenic recombination in rad22 and rti1 single and double mutant crosses. Interval analysed ade1-40 lys4-95 leu2-120 lys7-2 a Average number Genotype of the Average RF of colonies parents and value ± SEMa analyzed in each strain experiment (%) designations Average genetic distance (cM)b Relative average genetic distance (%)c rad22+ rti1+ (1-11×3-99) 24.1 ± 1 384 33.4 ± 1.8 100 rad22 (GO123×GO126) 25.1 ± 1.5 384 34.9 ± 2.6 100 rti1 (GO179×GO181) 24.4 ± 1 384 33.6 ± 1.7 100 rad22 rti1 (GO228×GO243) 20.5 ± 0.8 320 26.5 ± 1.4 79.3 rad22+ (3-87×4-150) 10.9 ± 0.7 384 12.2 ± 0.9 100 rad22 (GO124×GO164) 12± 0.4 384 13.7 ± 0.4 100 Recombination frequency (RF) was calculated as the percentage of recombinant colonies among all colonies analyzed. Mean and standard error of the mean (SEM) were obtained from three independent crosses. b Genetic distance in centiMorgans (cM) was calculated as indicated in Material and Methods. c Values are relative to rad22+ level, which is set to 100%. - 10 - Table A5: Values of intragenic recombination in rad22 and rti1 single and double mutants. Interval analysed Range of number of Genotype of the prototrophs Relative average Average ppm parents and strain analyzed in each ppm value ± SEMa designations experiment (%)b rad22+ rti1+ 866 - 1029 100 380 ± 29 (5-167×6-216) rad22 303 - 406 39.5 150±12 ade6-M375 (GO165×GO214) ade6-469 rti1 360±17 851 - 1036 94.7 (GO170×GO173) rad22 rti1 298 - 396 65.8 250±13 (GO218×GO232) rad22+ 973 - 1127 100 610 ± 30 (1-26×51-2009) rad22 270±15 367 - 431 44.3 (GO127×GO211) ade7-50 ade7-152 rti1 680±97 911 - 1414 100 (GO175×GO177) rad22 rti1 460±35 692 - 780 75.4 (GO215×GO229) a Prototrophs per million (ppm) were calculated as the number of prototrophic colonies per million viable spores plated. Spore viability was determined by plating the spores on rich medium (supplemented YEA). Mean and standard error of the mean (SEM) were obtained from three independent crosses. b Values are relative to rad22+ level, which is set to 100%. - 11 - Table A6: Values of intrachromosomal recombination in rad22 and rti1 mutants. Genotype of the parents and strain designations a Range of prototrophs Relative average Average ppm value ± scored per experiment ppm value SEMa (%)b rad22+ rti1+ (MR61×MR62) 80800 ± 2000 2418 - 3228 100 rad22 (GO271×GO285) 13000 ± 2500 188 - 570 16 rti1 (GO273×GO288) 23800 ± 3900 630 - 1111 29 rad22 rti1 (GO267×GO284) 1000 ± 300 1-3 1 Mean and standard error of the mean (SEM) for three independent crosses. Prototrophs per million (ppm) were calculated as the number of prototrophic colonies per million viable spores plated. Spore viability was determined by plating the spores on rich medium (supplemented YEA). b Values are relative to wild-type level, which was set to 100%. - 12 - SUPPLEMENTAL FIGURE LEGENDS Figure A1: Comparison of amino acid sequences of Rad52 homologs. The alignment was realized using the T-COFFEE algorithm. The level of identity between the different regions of the proteins is indicated by colours, ranging from blue for “different” to red for “identical”. Gaps in which no alignment could be determined are marked with dashes (-). The line below the alignment symbolizes the levels of overall amino acid conservation between the proteins, ranging from highly conserved ones marked with a star (*) to slightly conserved ones marked with a single dot (.). Figure A2: Genotoxin sensitivity of rad22 mutant strains. Suppressed (*) and non-suppressed rad22 deletion strains were cultured and serially diluted. The leftmost spot in each panel represents 105 cells plated. Strains plated in the rows 5 to 7 were obtained by back-crossing RGL6, RGL10 and RGL13 (rows 2 to 4) with a wild type strain. rad22 and rad22 rti1 strains display high sensitivity, which can be suppressed by the deletion of the fbh1 gene (OSMAN et al. 2005). Figure A3: Immunostaining of whole mitotic cells. White arrows indicate the positions of the nuclei. (A) wild type, rad22, and rad22 rti1 strains were stained with DAPI (left panels) and anti-Rad22 antibody (right panels). A nuclear signal for Rad22 was detected. (B) A wildtype strain was stained with DAPI (left panels) and anti-Rti1 antibody (right panels). No signal could be detected in the nucleus. Figure A4: Preliminary scheme on the role of Rad22 in avoidance of a lethal lesion possibly occurring at the mating-type locus. Rad22 is proposed to act at the pausing replication fork at - 13 - the smt site of mat1. Single-strand invasion by a Rad51-coated DNA filament into one of the silent cassettes (mat2 or mat3) on the same or the homologous chromosome, or into mat1 of the homologous chromosome, is proposed to require Rad22. In the absence of Rad22, a lethal DNA lesion is proposed to occur. In the absence of functional smt sequence, the imprint (arrowhead) is not formed, as well as the lesion, resulting in survival of the rad22 spore. - 14 -