How Ions Are Formed
advertisement

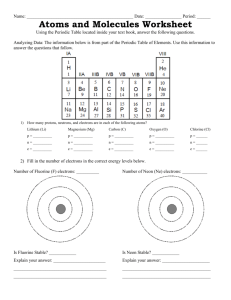
CP Biology Name _________________________________________________ Date ________________Per_______ Activity: How Ions Are Formed Objective: In this activity you will be building ions of biologically important elements. Introduction: You have learned that a molecule is a compound that is composed of atoms joined by covalent bonds. Covalent bonds form when atoms share electrons. They are strong bonds that help to form many different compounds. Proteins, the large organic molecules necessary for life, are held together by strong covalent bonds. Another type of compound important in biological systems is the ionic compound. Ionic compounds differ from molecular compounds because they are made of atoms called ions. Ion-An atom that has either lost or gained one or more electrons in order to become stable: a) When an atom loses electrons to become stable, it becomes an ion with a ___________ charge. b) When an atom gains electrons to become stable, it becomes an ion with a ___________ charge. Ionic compounds are made of positive and negative charged ions that have been attracted to each other. When ions are mixed in water, they separate or dissociate from each other. The resulting ions are then available for use in different body processes. Several important ions in the human body: Ion Calcium Iron Use in the body: Ca2+ Fe2+ Sodium Na+ Important in the formation of bones and in the clotting of blood. Used to build hemoglobin, the molecule that carries oxygen in blood cells. Found in and out of all cells in the body. Used in nerve cells. Potassium K+ As above. Cl- As above. Chloride Magnesium Mg2+ An important compound that helps metabolic reactions take place. What types of ions will be formed from the elements above? Will they form ions with a positive or negative charge? How many electrons will they gain or lose? Procedure: Answer the following questions to determine what types of ions will be formed from the above compounds. 1) What charge does one proton have? __________________________________ 2) What charge does one electron have? _________________________________ 3) How many protons does an atom of carbon have? _______________ How many electrons? _____________ 4) Explain why atoms in their elemental form, like carbon above, do not have a charge and are neutral. ______________________________________________________________________________________________ Collect the following materials: paper clips to represent electrons pennies to represent protons chalk to draw the nucleus and energy levels The Periodic Table of the Elements Use the chalk to draw a small circle about the size of your hand in the center of your desk. This will represent the nucleus of an atom. You will draw energy levels around the nucleus depending upon the number of electrons in the atom you are asked to draw. 5) Build the atom called Fluorine: a) What is the atomic number of Fluorine? _________ How many protons does Fluorine have? ___________ Place this number of pennies inside the nucleus you have drawn b) How many electrons does Fluorine have? _______________ Count this number of paper clips to represent electrons. c) How many electrons will be in the first energy level of Fluorine? ________________ Draw a circle around the nucleus to represent the first energy level. Place paper clips representing the correct number of electrons on the circle. d) How many electrons will be in the second energy level of Fluorine? ______________ Draw a circle around the nucleus to represent the second energy level. Place paper clips representing the correct number of electrons on the circle. e) Will there be electrons in the third energy level? _________________ 6) Look at the atom of Fluorine that you have created. Is it stable and non-reactive or is it unstable and reactive? How do you know? ______________________________________________________________________________ ______________________________________________________________________________________________ 7) In order to become stable would it be easier for Fluorine to lose 8 electrons or gain one electron? ______________ (Keep in mind that it is difficult to move larger numbers of electrons.) 8) Add or remove the correct number of paperclips (electrons) to make a stable Fluorine ion. 9) Now, count the number of pennies and paperclips in the Fluorine ion. Number of pennies (protons) _________ Number of paperclips (electrons) ______________ 10) Do you have more protons or electrons? ________________ How many more? _______________ 11) How many positively charged particles are in the Fluorine ion? _____________ Negative particles? _____________ 12) What is the charge of the Fluorine ion? (positive, negative) _______________________________ 13) We write the chemical formula for Fluorine as F-. This shows that the ion carries one more electron than protons. If an ion has two more electrons, it is written with a superscript of 2- . Three electrons in an ion are shown with 3-. 14) Ions can also lose electrons to become stable. An ion with one more proton than electrons is written with a superscript of _____________. An ion with two more protons is written with a superscript of _________. Calcium forms a positive ion written as Ca2+. 15) Repeat the above exercise for each of the following atoms and fill in the chart. Atom H Mg Cl Na # protons # electrons # e in outer energy level will atom lose or gain an electron to become stable? How many? remaining proton(s) (pennies) in the ion remaining electron(s) (paperclips) final charge of ion chemical formula for ion Note: Hydrogen is composed of only one proton and one electron. Its electron is easily pulled away by an atom like oxygen. Hydrogen usually forms a positive ion called a hydrogen ion (H +) or, more simply, a proton. Ionic Bonding An ionic bond is formed when one or more electrons are transferred from one atom to another. The oppositely charged ions have a strong attraction, forming an ionic bond. Water easily dissolves ionic compounds. These two ions are now attracted to eachother…that’s an ionic bond!