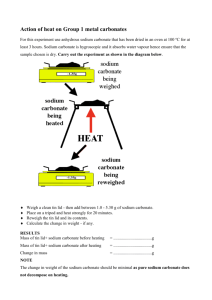
Action of heat on transition metal carbonates In
advertisement
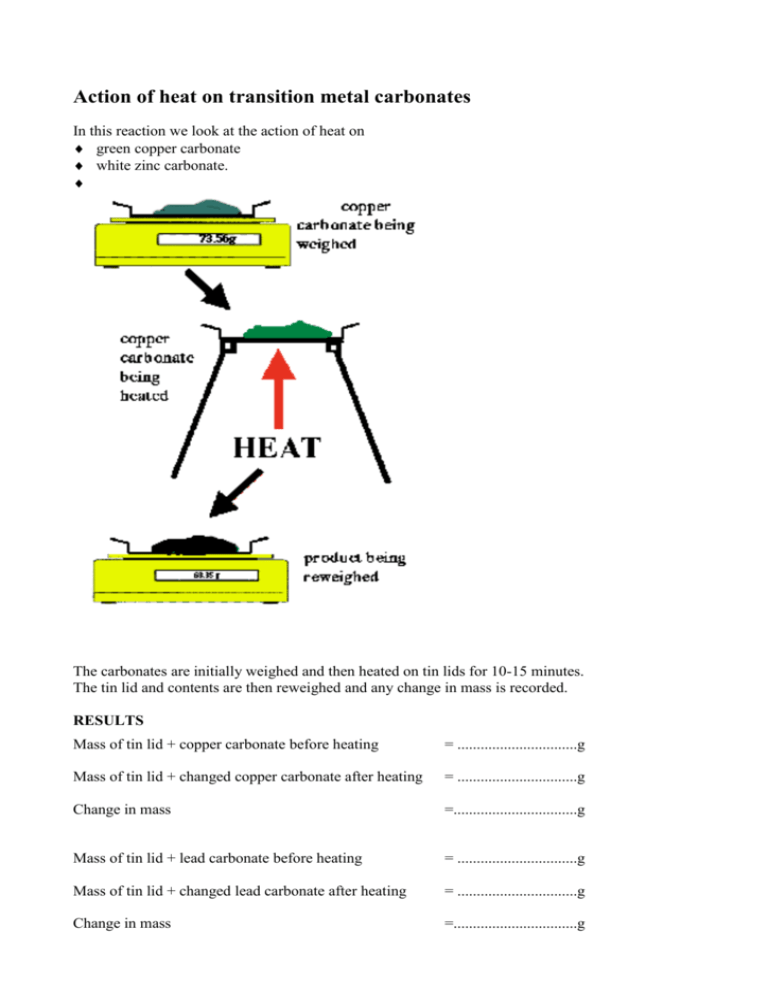
Action of heat on transition metal carbonates In this reaction we look at the action of heat on green copper carbonate white zinc carbonate. The carbonates are initially weighed and then heated on tin lids for 10-15 minutes. The tin lid and contents are then reweighed and any change in mass is recorded. RESULTS Mass of tin lid + copper carbonate before heating = ...............................g Mass of tin lid + changed copper carbonate after heating = ...............................g Change in mass =................................g Mass of tin lid + lead carbonate before heating = ...............................g Mass of tin lid + changed lead carbonate after heating = ...............................g Change in mass =................................g WHAT HAPPENS When green copper carbonate is heated carbon dioxide gas and black copper oxide are produced. When white lead carbonate is heated carbon dioxide and white lead oxide (yellow when hot) are produced. These carbonates have split up into simpler substances. They have DECOMPOSED. The equation for these reactions can be written simply as: Action of heat on copper carbonate Copper carbonate Copper oxide green black CuCO3 CuO + CO2 Lead oxide + carbon Action of heat on lead carbonate Lead carbonate white white PbCO3 PbO DANGERS LEAD CARBONATE TOXIC (POISONOUS) – Any material swallowed may cause severe injury. Seek medical attention COPPER CARBONATE IRRITANT – Dust may irritate the lungs and eyes. + carbon dioxide gas dioxide gas + CO2