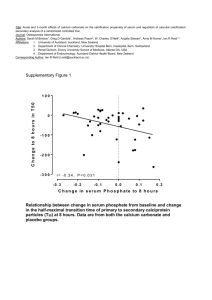
Comparing the Calcium Content of Brown and White
advertisement

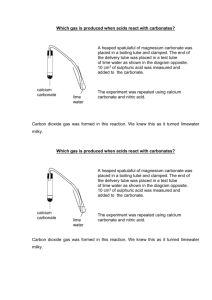
Determining the Calcium Content of Eggshells Purpose: To determine the calcium carbonate content of eggshells Background: Calcium carbonate is a component of seashells and eggshells that gives them their strength and hardness. When calcium carbonate reacts with hydrochloric acid, the products are carbon dioxide, water, and calcium chloride. CaCO3 (s) + HCl (ag) CaCl2 (aq) + CO2 (g) + H2O (l) You will be adding hydrochloric acid to an eggshell to dissolve all of the calcium carbonate in the shell. The portion of the eggshell that is not calcium carbonate does not react with the acid and remains a solid. You will be separating the solid parts of the eggshell from the calcium carbonate by filtration. This will allow you to determine the percent of the eggshell that was calcium carbonate. Safety: You will be working with concentrated hydrochloric acid. Goggles must be worn at all times during this lab. If you get any of the acid on your skin wash your hands with soap and a lot of water. Procedure: 1. Obtain a piece of eggshell and crush it. Transfer the eggshell to a 250 ml beaker and find its mass. 3. Add 45 ml of 1.0 M HCl to the eggshell and stir with a glass stirring rod until there are no more bubbles forming. 4. Find and record the mass of a dry piece of filter paper. Write your name and period number on the filter paper with a pencil. Fold the filter paper and put it in the funnel. Set the funnel over an Erlenmeyer flask. 5. Pour the dissolved eggshell and acid through the filter. Wash the filtrate with a little deionized water. 6. Put your filter paper in an 80 ml beaker with your name on it and bring it to the oven to dry until tomorrow. When the filtrate is dry find its mass. Calculations: - Calculate the mass of the filtrate by subtracting the mass of the filter paper from the mass of the filtrate and the filter paper. - Calculate the mass of the calcium carbonate by subtracting the mass of the filtrate from the mass of the eggshell. - Calculate the percent of calcium carbonate in the eggshell by dividing the mass of calcium carbonate by the mass of the eggshell and then multiplying by 100. - Calculate the number of moles of calcium carbonate in your sample. - Calculate the number of formula units of calcium carbonate in your sample.