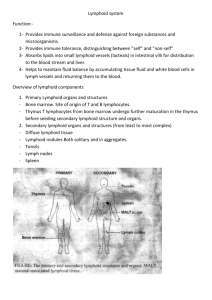
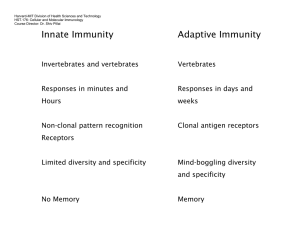
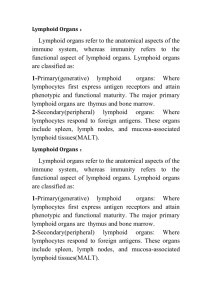
Formation of tertiary lymphoid tissue in dextran sulfate
advertisement

Formation of tertiary lymphoid tissue in dextran sulfate sodium induced colitis is partially dependant on LT1 2-LTR axis B.J. Olivier1, M. Knippenberg1, M.J. Greuter1, G. Goverse1, E.D. Keuning1, A.A. te Velde2, G.Bouma3 and R.E. Mebius1. 1 Department of Molecular Cell Biology and Immunology, Vrije Universiteit Medical Center, Amsterdam, The Netherlands. 2 Tytgat Institute for Liver and Intestinal Research, Academic Medical Center, Amsterdam, The Netherlands. 3 Department of Gastroenterology, Vrije Universiteit Medical Center, Amsterdam, The Netherlands. Patients with inflammatory bowel disease (IBD) suffer from chronic inflammation of the intestine, which may lead to the formation of lymphoid aggregates that closely resemble secondary lymphoid tissue. The formation of secondary lymphoid tissue is dependent on the lymphotoxin alpha1 beta2 - lymphotoxin beta receptor signaling axis. Hematopoietic lymphoid tissue inducer (LTi) cells express lymphotoxin alpha1 beta2 (LT12) which binds to the lymphotoxin beta receptor (LTR) expressed on stromal organizers cells, and leads to the induction of chemokines (CXCL13 and CCL21) and adhesion molecules (VCAM1, ICAM and MAdCAM-1). These molecules serve to attract and retain additional hematopoietic cells leading to the formation of secondary lymphoid tissue. Here we show, using dextran sulfate sodium (DSS) induced colitis, that stromal cells in the acute inflammatory setting have the ability to upregulate LTR expression along with CXCL13, CCL21, VCAM-1 and MAdCAM-1. In the more chronic setting tertiary lymphoid tissues were detected in inflamed colons and consisted of tightly clustered B cell follicles with distinct T cell areas. Surprisingly, these structures could also be found at the chronic phase of DSS colitis in LT-/- mice, although the B cell areas were devoid of follicular dendritic cells (FDCs) and germinal centers (GCs). These results show that lamina propria stromal cells become activated upon damage to the epithelial barrier and function as organizer cells to locally form lymphoid tissue. This process is only partially dependent on the LT12-LTR axis and must involve alternate pathways.