Molecular Structure Worksheet: Lewis Structures & VSEPR
advertisement
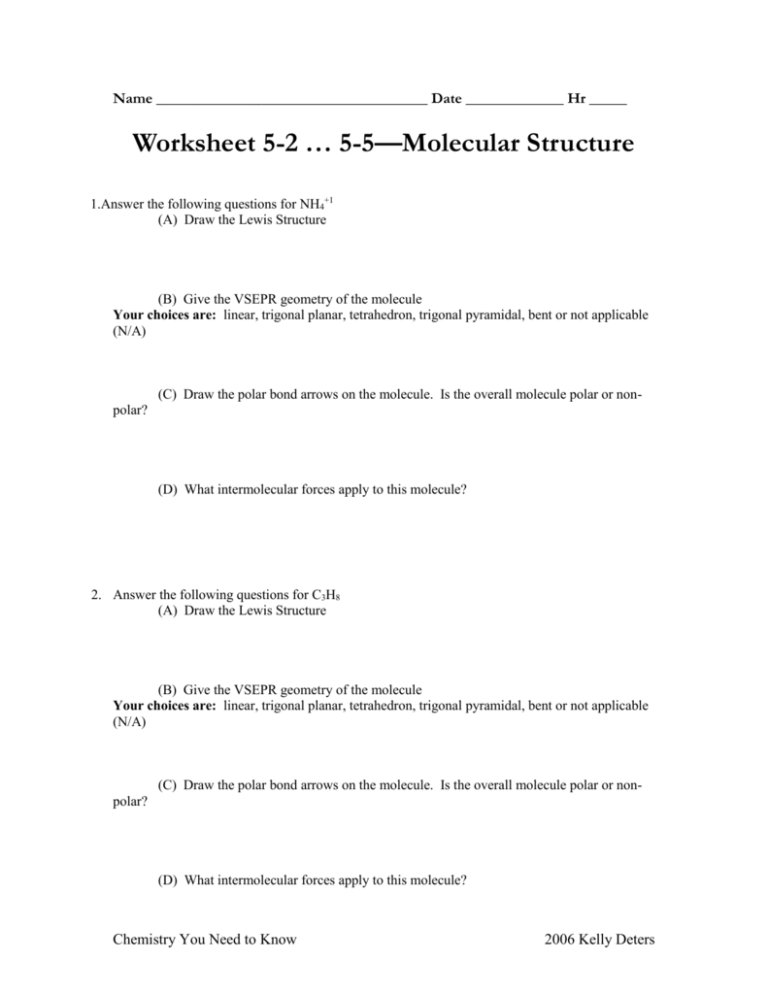
Name ____________________________________ Date _____________ Hr _____ Worksheet 5-2 … 5-5—Molecular Structure 1.Answer the following questions for NH4+1 (A) Draw the Lewis Structure (B) Give the VSEPR geometry of the molecule Your choices are: linear, trigonal planar, tetrahedron, trigonal pyramidal, bent or not applicable (N/A) (C) Draw the polar bond arrows on the molecule. Is the overall molecule polar or nonpolar? (D) What intermolecular forces apply to this molecule? 2. Answer the following questions for C3H8 (A) Draw the Lewis Structure (B) Give the VSEPR geometry of the molecule Your choices are: linear, trigonal planar, tetrahedron, trigonal pyramidal, bent or not applicable (N/A) (C) Draw the polar bond arrows on the molecule. Is the overall molecule polar or nonpolar? (D) What intermolecular forces apply to this molecule? Chemistry You Need to Know 2006 Kelly Deters 3. Answer the following questions for CO3-2 (A) Draw the Lewis Structure (B) Give the VSEPR geometry of the molecule Your choices are: linear, trigonal planar, tetrahedron, trigonal pyramidal, bent or not applicable (N/A) (C) Draw the polar bond arrows on the molecule. Is the overall molecule polar or nonpolar? (D) What intermolecular forces apply to this molecule? 4. Answer the following questions for H2O (A) Draw the Lewis Structure (B) Give the VSEPR geometry of the molecule Your choices are: linear, trigonal planar, tetrahedron, trigonal pyramidal, bent or not applicable (N/A) (C) Draw the polar bond arrows on the molecule. Is the overall molecule polar or nonpolar? (D) What intermolecular forces apply to this molecule? 6. Draw the following ionic Lewis Structure: CaF2 Chemistry You Need to Know 2006 Kelly Deters








