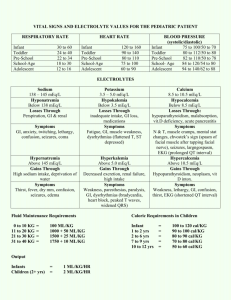
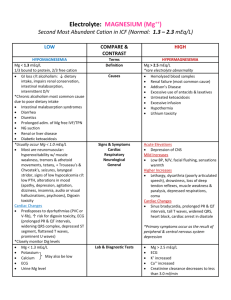
Quantative Composition
advertisement

Quantative Composition Formula when powder dissolved in water: Anhydrous calcium lactate, 0.0436 g: anhydrous magnesium sulphate, 0.024 g: anhydrous citric acid, 0.032 g: anhydrous sodium citrate, 0.258g: anhydrous sodium chloride, 0.0585 g: anhydrous potassium chloride, 0.149 g: anhydrous sodium phosphate, 0.071 g: anhydrous glucose, 5g. Additives: Neohesperdine, Orange flavour and yellow-orange colouring (E-110) Formula of the solution expressed in mEq: Lactate 0.4 mEq; Sulphate 0.4 mEq; Citrate 3.5 mEq; Phosphate 1 mEq; Chloride, 3 mEq; Calcium 0.4mg; Sodium 5 mEq; Magnesium 0.4 mEq; Potassium 2mEq; anhydrous glucose 5g (20 Calories). Additives: Neohesperdine, Orange flavour and yellow-orange colouring (E-110) Indications: Acute diarrhoea in babies; as a prophylaxis and treatment for states of moderate dehydration; acidosis or vomiting; in extra-renal uremia and in general for all cases of profuse fluid loss through persistent diarrhoea or vomiting; hyperemesis gravidarum etc Posology: To be taken orally. In drink form, until complete rehydration is judged to be achieved. Counterindications and Precautions: Must be used with caution in diabetics and patients with parasitic infestations. Serious or persistent diarrhoea, or other critical losses of fluids which require parenteral therapy: continuous vomiting, paralytic ileus, intestinal obstruction or perforation; diminished renal function (anuria, oliguria). Warning This medicine contains 5g of anhydrous glucose in every 100ml of reconstituted solution, which must be taken into account when dealing with diabetic patients. Warning re: additives The medicine contains yellow orange colouring S (E-110) as a non-clinical additive. It can cause allergic reactions including asthma, especially in patients allergic to Acetylsalicylic acid. Incompatibilities It is not advisable to mix CITORSAL with milk, fruit juice or other liquids containing electrolytes; if the administration of more liquid is required, water should be used. The product interferes with the absorption of tetracycline. Side effects Used at recommended dilution, no side effects have been described. Overdose and its treatment There have been no known cases of overdose in this product. In case of overdose or accidental ingestion, please consult the Servicio de Informacion Toxicologica Tel 91/562-04-20 Presentation and Prices Pack containing 2 sachets of soluble powder P.V.P. IVA 1.69E C.N. 853499 Pack containing 5 sachets of soluble powder P.V.P. IVA 3.73E C.N. 827378 Instructions for correct administration Each sachet should be dissolved in ½ litre of water: or two sachets in one litre. To make the dissolution process easier, we recommend the powder is added slowly to the water whilst vigorously stirring until it has completely dissolved. In certain conditions, which have no effect on the products therapeutic qualities, CITORSAL may harden in the packet. In this case, simply press on the bag until the CITORSAL is reduced to a fine, soluble powder. Conditions of Prescription and Dispensation With medical prescription. Can be financed by S.N.S. S.N.S subsidy at normal rate. Laboratorios ERN SA Pedro IV, 499-08020 Barcelona, España