PARTICIPANT INFORMATION LEAFLET
advertisement

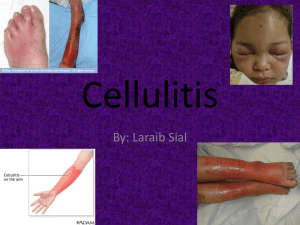
Local NHS Trust heading Randomised controlled trials to investigate whether prophylactic antibiotics can prevent further episodes of cellulitis (erysipelas) of the leg (PATCH I & PATCH II) PARTICIPANT INFORMATION SHEET You are being invited to take part in a research study. This will not involve any changes to the treatment that you receive whilst in hospital. Before you decide whether or not to take part, it is important for you to understand why we are doing this research and what we will ask you to do if you agree to help us. Please take time to read the following information carefully and discuss it with others if you wish. Ask us if there is anything that is not clear or if you would like more information. Thank you for reading this. What is the purpose of the study? Cellulitis is an acute, painful and potentially serious infection of the skin and underlying tissue. We want to find out whether taking a low dose of penicillin every day for six or twelve months helps to prevent further episodes of cellulitis of the leg in patients who have previously suffered from this. What is the plan of the study? We will ask all participants to take a tablet every morning and every evening for twelve months (PATCH I trial) or for six months (PATCH II trial). Half of the participants in each trial will be given tablets containing a low dose of penicillin (an antibiotic which has been used for treating infections for many years), and the other half will be given ‘dummy’ tablets which contain no active ingredients at all (called a ‘placebo’). After the end of the study period we will compare the number of repeat episodes of cellulitis in each group of patients and will then find out whether the penicillin helped to prevent further episodes. This kind of research study is called a ‘randomised controlled trial’. Why have I been chosen? You have been asked to help with this research because you have had an episode of cellulitis of your leg (an infection of the skin). We are asking all patients, like you, who have been treated in this hospital for cellulitis of the leg to help with our research. Do I have to take part? It is up to you to decide whether or not to take part. If you do agree to take part you will be given this information sheet to keep and be asked to sign a consent form (a copy of which you will keep). Once you have agreed to take part you are still free to change your mind and withdraw at any time and without giving any reason. A decision not to take part or withdrawal from the study will not affect the standard of care or treatment that you receive, either now or at any time in the future. 1 PATCH STUDY EudraCT No 2006-000381-36 Participant Information Leaflet v1.3 180906 What will happen to me if I decide to take part? If you agree to help with this study, a doctor or nurse will arrange to see you at the hospital. S/he will ask you a few simple questions about your medical history and will want to know about your cellulitis. You will have the opportunity to ask any questions and discuss any concerns you may have. Then, if the doctor or nurse thinks you are suitable for the study and you are still willing to take part, you will be asked to sign a consent form. You will probably already have had some simple blood tests done when you were seen with your episode of cellulitis, but if not, then we will take a blood sample. If this should result in a finding which needs further investigation, then this will be arranged. A lot of the information that we need can be obtained from your medical notes, so the doctor or nurse will ask you if they can look at your hospital and GP notes for the purposes of this research. If you agree to take part, you will carry on with your treatment as normal until your cellulitis has cleared up. What do I have to do? Within a few days you will receive at home through the post a supply of tablets, with instructions to take one every morning and one every evening. You will not have to make any changes to your usual lifestyle or your diet, and you won’t have to avoid alcohol. You will not be able to choose whether you are in the group taking the ‘active’ tablets or the group taking the ‘dummy’ tablets, as this selection will be made randomly by a computer. There is a 1 in 2 chance that you will receive the active treatment but neither you nor the research doctor will know whether the tablets you are given are the ‘active’ or the ‘dummy’ tablets until after the end of the study. This is so that no-one involved in the trial can influence the results. We will inform your own GP that you have agreed to take part in this research study and will ask your permission to approach him/her for information from your medical records. If your doctor should need to find out for medical reasons which treatment you are taking, s/he can do so. You will be asked to take one tablet twice a day for six months or for twelve months, depending on whether you are in the PATCH I or the PATCH II trial. During this time the researchers will contact you by telephone every now and then to see if you are having any problems and to see if you have suffered from another episode of cellulitis. The researchers will also ask to contact your GP in order to check for information in your medical notes. We will give you a diary so that you can keep a record of when you have taken the medication, any symptoms you get, and whether you have a further attack of cellulitis. At the end of the chosen treatment period (six months or twelve months) you will be asked by telephone to stop taking the tablets, but we will continue to contact you every so often for up to two years. You will not have to go anywhere or do anything other than answer a few simple questions about your health over the telephone. What if I am allergic or have had a bad reaction to penicillin? It should already be known if you are allergic because you have had treatment for cellulitis in the past. However, if there is any uncertainty, you will be able to discuss this with the doctor/nurse when s/he talks to you about the trial. Patients with an allergy to penicillin are not suitable for our study and will therefore not be asked to take part. 2 PATCH STUDY EudraCT No 2006-000381-36 Participant Information Leaflet v1.3 180906 What if the tablets make me feel unwell? It is very unusual for people to have any side-effects from the low dose of active tablets which is given, as long as they are careful to follow the instructions about how to take them. Very occasionally however, some people may get slight digestive discomfort or some looseness of stools. You will be given a telephone contact number to ring if you have any worries about this. There are a number of conditions that could be related to long-term use of penicillin. These include inflammation of the tongue or mouth; thrush or discharge from the vagina; blood disorders causing symptoms of tiredness, sore throat, easy bruising and susceptibility to infection. If you decide to take part in the study, we will advise you what to do if you get any symptoms. Will the tablets react with my normal medication? There are very few medications that would be affected by taking penicillin at the same time. When you discuss the trial with the doctor/nurse, s/he will ask about any other treatment you are on and you will not be asked to take part if there is a possibility that the trial medication would interfere with any on-going treatment. Will taking penicillin tablets for a long time mean that they won’t be effective if I have to take them for an illness in the future? No. This form of penicillin has been used for more than sixty years now and is used as long-term medication (continuing for several years) in patients with other conditions, without them becoming resistant to it. What if something goes wrong? There are no special compensation arrangements for this study. However, if you are harmed as a result of someone’s negligence, then you may have grounds for a legal action but you may have to pay for it. Regardless of this, if you wish to complain about any aspect of the way you have been approached or treated during the course of this study, the normal National Health Service complaints mechanisms are available to you Patient Advice and Liaison Service (PALS). Will my taking part in the study be kept confidential? All information that is collected about you during the course of the research will be kept strictly confidential and stored in a locked room. This information will be used to analyse the results of the study, and will be kept for 5 years after the end of the study. Who is organising the research? The research is being organised through the UK Dermatology Clinical Trials Network (www.ukdctn.org). This is a group of skin doctors and nurses who have agreed to work together in order to research important questions of relevance to both doctors and patients. None of the doctors or nurses helping with this study will receive any payment other than for administrative costs. The information collected will be analysed by researchers employed at the University of Nottingham. Who is funding the research? The work is being funded by two different charities: Action Medical Research (www.action.org.uk) and The BUPA Foundation (www.bupafoundation.com), with additional support from the UK Dermatology Clinical Trials Network (www.ukdctn.org). Will I be paid for taking part in this study? No, because this is charity-funded research, participants will not be paid for taking part in the study. 3 PATCH STUDY EudraCT No 2006-000381-36 Participant Information Leaflet v1.3 180906 Who has reviewed the study? The plan for the study has been looked at and approved by several consultants who specialise in treating skin problems (dermatologists), and also by a group of patients who have themselves experienced cellulitis. We have received approval from the government’s ethical committee for the study (MREC No 06/Q2404/22) and approval from your local hospital’s ethics committee. Contact for further information If you have any questions about this research, please contact: Local contact details Dr Tel. Alternatively, you may prefer to contact the researchers who will be analysing the data at the University of Nottingham. If so, please contact: PATCH Trial Manager UK Dermatology Clinical Trials Network King’s Meadow Campus University of Nottingham Nottingham NG7 2NR Tel: 0115 846 8626 Fax: 0115 846 8618 email: patch@nottingham.ac.uk 4 PATCH STUDY EudraCT No 2006-000381-36 Participant Information Leaflet v1.3 180906