Beer`s Law I: Determining the Concentration of a Cobalt(II) Chloride
advertisement

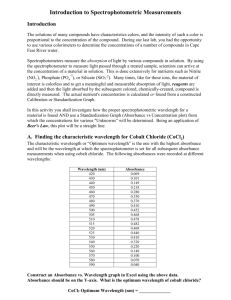
Beer’s Law I: Determining the Concentration of a Cobalt(II) Chloride Solution Introduction One of the most effective uses of the Spec-20D® spectrophotometer is in determining the concentration of an unknown solution. This technique can be used in forensics to determine the amount of poison in a murder victim’s tissues, in pharmacology to determine the safe and effective dosage of a new drug, or in industry to determine the con-centration of pollutants in wastewater. This spectrophotometric technique makes use of the Beer-Lambert law, frequently known as “Beer’s Law.” We represent the Beer-Lambert law as A = abc where A is the absorbance, a is the molar absorptivity of the substance in question, b is the length of the light path inside the spectrophotometer, and c is the concentration of the solution. In this experiment, we will apply the Beer-Lambert law to a series of aqueous solutions of cobalt(II) chloride, CoCl2 , which have a pink color due to the presence of Co 2+ ions. We will identify the wavelength of maximum ab-sorbance in the absorption spectrum of Co2+, then use that wavelength in determining the absorbances of a series of solutions of known concentration. When we plot the measured absorbances against concentration, we will observe a linear relationship; the slope of the line will correspond to ab. Since the path length, b, can be measured, we can calculate the value of a. As long as the wavelength is not changed, a will be a constant for any solution of CoCl2 . Thus, we can measure the ab-sorbance of a solution of Co2+ and calculate its concentration from the information we already have. We report the concentration as “[Co2+] = x”; this is notation you will see frequently in future labs. This may all sound terribly complex, but it really isn’t. And to make matters better, obtaining the absorption curve is something you only have to do once for each substance. Objectives By doing this lab, students will be able to: state the Beer-Lambert law and define each variable in it use an absorption curve to determine the concentration of Co 2+ in a solution of unknown concentration extrapolate from what they learn in this lab to determine the concentrations of ions in other solutions Materials and Equipment 0.15 M cobalt(II) chloride solution cobalt(II) chloride solution, concentration unknown 6 cuvettes stirring rod Eppendorf® micropipetter graph paper Spec-20D spectrophotometer ruler Safety Considerations As always, you should wear safety glasses or goggles when working in the lab area. Cobalt(II) chloride is toxic by ingestion. If you have open wounds on your hands, you should wear gloves during this experiment in order to prevent blood damage. Avoid dropping or breaking the cuvettes – they can be quite expensive. Procedure A: Obtaining the Absorption Spectrum Turn on the Spec-20D and allow it to warm up. This takes about ten minutes; your instructor may have already done this for you. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Prepare a “blank” cuvette by placing 5.0 mL of distilled/deionized water into a cuvette. Obtain approximately 20 mL of 0.15 M cobalt(II) chloride solution. Using the micropipetter, transfer 5.0 mL of the cobalt(II) chloride solution to another cuvette. Using the large knob on top of the Spec-20D, adjust the wavelength to 400 nm (nanometers). The light next to TRANSMITTANCE should be lit; if it is not, press the MODE button until it is. With nothing in the sample compartment and the lid closed, set the transmittance to zero using the front left knob. Press the MODE button once so that the light next to ABSORBANCE is lit. Place the blank cuvette in the sample compartment and close the lid. Set the absorbance to zero using the front right knob. Remove the blank and replace it with the cuvette containing the cobalt(II) chloride solution. Close the lid. Record the absorbance on the data table. Change the wavelength to 425 nm and repeat Steps 8 – 10. Continue to change the wavelength by 25 nm and repeat Steps 8 – 10 until you reach 600 nm. Remember that each time you change the wavelength, you must adjust the absorbance to zero using the blank. Graph the absorbance (A) on the y-axis versus wavelength on the x-axis. Draw a smooth curve to fit the ex-perimental points. Identify the maximum in the absorption curve to the nearest multiple of 25 nm and record this on your data sheet. Procedure B: Validating the Beer-Lambert Law 1. Using the micropipetter, prepare a series of cobalt(II) chloride solutions using the following table. Do all the CoCl2 transfers before adding water to any of the cuvettes; this means you use only two micropipetter tips in the preparation. Mix the contents of each cuvette using a clean, dry stirring rod. Note: You prepared tube 1 in Procedure A. Keep in mind that careful measurement is important! Tube # 1 2 3 4 5 Volume of 0.15 M CoCl2 (mL) 5.0 4.0 3.0 2.0 1.0 Volume of distilled water (mL) 0.0 1.0 2.0 3.0 4.0 2. Set the wavelength of the Spec-20D to the wavelength of maximum absorbance you found in Procedure A. Use the blank cuvette to adjust the absorbance to zero. 3. Measure and record the absorbances of tubes 2 through 5 (you already have the absorbance for 4. 5. tube 1). Calculate the concentration of each of the solutions as a percentage or ratio of tube 1. Graph the solutions’ absorbances versus [Co2+]. Use a ruler to draw a straight line through the origin and as close as possible to all the experimental points. Calculate the slope of this line. Procedure C: Determining the Concentration of an Unknown Solution Obtain about 5 mL of a cobalt(II) chloride solution of unknown concentration and record its letter/number on your data sheet. 1. Place the sample in the Spec-20D and record its absorbance on your data sheet. 2. Calculate the concentration of the unknown using Beer’s Law and the data you gathered earlier (see the sidebar below for help). Disposal and Cleanup Pour your cobalt(II) chloride cuvettes into the waste container provided by your instructor. Pour the distilled water down the drain. Thoroughly rinse out the cuvettes with tap water and dry them with a Kimwipe or three. Wash your hands thoroughly with soap and water. Calculating the Concentration of the Unknown We can use the numbers we’ve obtained in the procedure to calculate the concentration of the unknown. Re-member that Beer’s Law is A = abc, where A = absorbance [from the Spec-20D] a = absorptivity, which is a constant for each individual compound = slope of line b = path length, which is a constant when using the same Spec-20D = 1 cm in this experiment c = concentration Comparing one standard (s) and one unknown (u), Since a and b are the same for both the standard and the unknown (and can be canceled out), If you’re worried about this, check the algebra yourself on a piece of scratch paper before you plug in the numbers.