Introduction to Spectrophotometric Measurements
advertisement

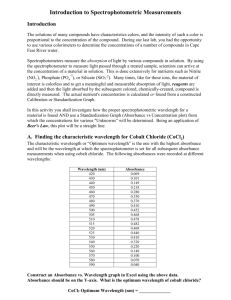
Introduction to Spectrophotometric Measurements (Use of the Spectrophotometer) Introduction By using the spectrophotometer to measure light passed through a treated sample, scientists can arrive at the concentration of a material in solution. This is done extensively for nutrients such -3 as Nitrate (NO3 ) or Phosphate (PO4 ). Many times, like for these two ions, the material of interest is colorless and to get a meaningful and measurable absorption of light, reagents are added and then the light absorbed by the subsequent colored, chemically-created, compound is directly measured. The actual nutrient's concentration is calculated or found from a constructed Calibration or Standardization Graph. In today's lab we shall investigate how the proper spec wavelength for a material is found AND a Standardization Graph (Absorbance vs Concentration plot) will be constructed from which the concentrations for various "Unknowns" will be found. Being an application of Beer's Law, this plot will be a straight line. A. Finding the characteristic wavelength for Cobalt Chloride (CoCl2) This will be done as a class activity. Operation of the spectrophotometer will be demonstrated and practiced. Absorbances at different wavelengths will be measured and graphed. The "Optimum wavelength" is the one with the highest absorbance and will be the wavelength at which the spectrophotometer is set for all subsequent absorbance measurements today. B. Creating the Standardization Graph: 1. From the stock Cobalt Chloride solution provided make up 20mL of a 50% V/V (volume to volume) solution. Use deionized distilled water as the diluent. 2. From the stock Cobalt Chloride solution provided make up 20mL of a 25% V/V (volume to volume) solution [25% CoCl2, 75% DIW]. Use deionized distilled water as the diluent. 3. Read the absorbance of these solutions, the Stock Cobalt Chloride solution, and record the values on your data sheet. 4. Construct an Absorbance vs. Concentration (%) graph from this data on the pre-labeled graph paper provided. Remember that the spectrophotometer blank is the 0% solution! Plot the four (4) data pairs, but use only the 100% and the 0% points to make your line. These standards were provided to you and are correct. The other two are of course your dilutions and their positions relative to this line will show how accurate your were in their creation. C. Using the Standardization Graph: 1. Using the same spectrophotometer on which you worked with your Standards (B1 & B2), measure the absorbances of the five (5) unknowns. One set is provided at each spec, you of course need to do only one set. Record these values on your data sheet. 2. Use your Absorbance vs. Concentration (%) graph (Standardization Graph) constructed in B to find the percent concentration of each unknown, recording this on your data sheet. Go into the graph with the Unknown's absorbance Read across to the line Mark the Unknown's Number [large enough to see!] on the line Its concentration is directly below! Additional Observations: (record the answers on your data sheet bottom) 3. What would be the absorbance of a 75% solution of this stock Cobalt Chloride? 4. What would be the absorbance of a 35% solution of this stock Cobalt Chloride? Due today: Your Data Sheet Your Standardization Graph Both should have your name and section on them. Please staple them together. Name: ______________________ Section: _____________________ Introduction to Spectroscopy Wavelength used for Cobalt Chloride Absorbances: _______nm Standards 100% (stock) 50% 25% 0% Unknowns 1 2 3 4 5 Absorbance ___________ ___________ ___________ ___________ Absorbance ___________ ___________ ___________ ___________ ___________ Additional Observations: 75% solution ___________ 35% solution ___________ % Concentration ___________ ___________ ___________ ___________ ___________